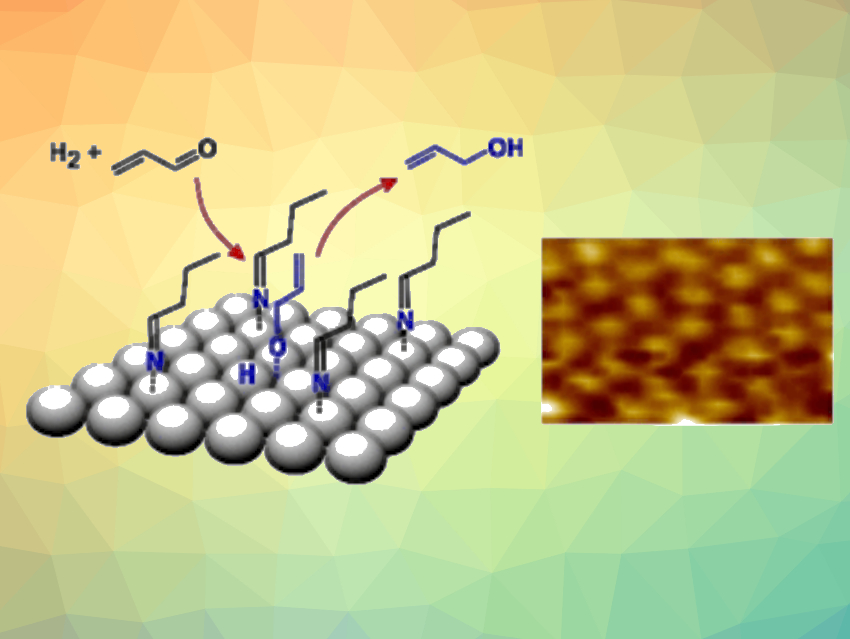
Chemical reactions do not always go to plan. Unwanted by-products lead to extra costs and wasted resources. Selective catalysts can help, but chemists have to test out large numbers before they find the right fit. Swetlana Schauermann, University of Kiel, Germany, and colleagues have investigated on an atomic level how to obtain a palladium catalyst for the selective hydrogenation of acrolein. The key appears to be a dense, convertible layer of ligand molecules.
The acrolein molecule has two positions where it can be hydrogenated. When reacted with hydrogen, either the alcohol propenol or the aldehyde propanal is formed. Palladium catalysts can be used to steer the reaction toward propenol, but scientists have observed that this only works if the surface of the metal has already been coated with the reaction partner or a similar hydrocarbon as a ligand precursor. The researchers investigated why this is the case and what actually happens in this reaction.
Ligand Layer on Solid Palladium Catalysts
For the team’s experiments, they first coated pure palladium metal with allyl cyanide, the ligand precursor for the reaction. To visualize this coating in detail, the researchers analyzed the palladium surface using scanning tunneling microscopy (STM). The results showed a “flat” coating with the allyl cyanide where all three carbon atoms of the allyl, as well as the cyanide functional group, lie flat on the metal atoms. No protrusions from the surface were noted.
This flat ligand layer changed when the metal was exposed to the reaction conditions and a stream of hydrogen was passed over the surface of the metal. Scanning tunneling microscopy revealed a dense coating, but with considerably shorter distances between the molecules. The researchers used the type of changes taking place, and spectroscopic analyses, to work out exactly what was going on. The hydrogen had hydrogenated the allyl cyanide molecule and converted it into a saturated hydrocarbon with an imine functional group.
The imine was no longer lying flat on the surface though: It was standing up. This happened because the end of the molecule with the saturated hydrocarbon residue had lost contact with the palladium atoms, while the imine function remained bonded to the metal. The flat surface of the catalyst had transformed into a forest of upright molecular trees.
Chemoselective Hydrogenation
This new coating activated the catalyst, enabling precise positional docking of the acrolein and activation of the oxygen function ready for hydrogenation. “On this active layer, acrolein nearly instantaneously forms the desired propenoxy reaction intermediate followed by evolution of the target product propenol,” the team observed.
The chemoselectivity and activity of the palladium catalyst could be explained in detail. “This is the first experimental proof of the formation of an active ligand layer obtained by real space microscopy,” state the researchers. The team hopes this new, deeper understanding could be used to find other functionalizations to improve the chemoselectivity of metal catalysts.
- Understanding Ligand‐Directed Heterogeneous Catalysis: When the Dynamically Changing Nature of the Ligand Layer Controls the Hydrogenation Selectivity,
Carsten Schröder, Marvin C. Schmidt, Philipp A. Haugg, Ann‐Katrin Baumann, Jan Smyczek, Swetlana Schauermann,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202103960