Cancer cells have a higher iron demand to support their faster proliferation rates. Several iron-binding compounds, such as thiosemicarbazone chelators, can exploit this vulnerability of malignant cells and are currently being investigated as anticancer drug candidates. Disulfide-based prochelators (“masked” chelators), for example, target intracellular iron pools because they are reductively activated for iron coordination upon cellular uptake.
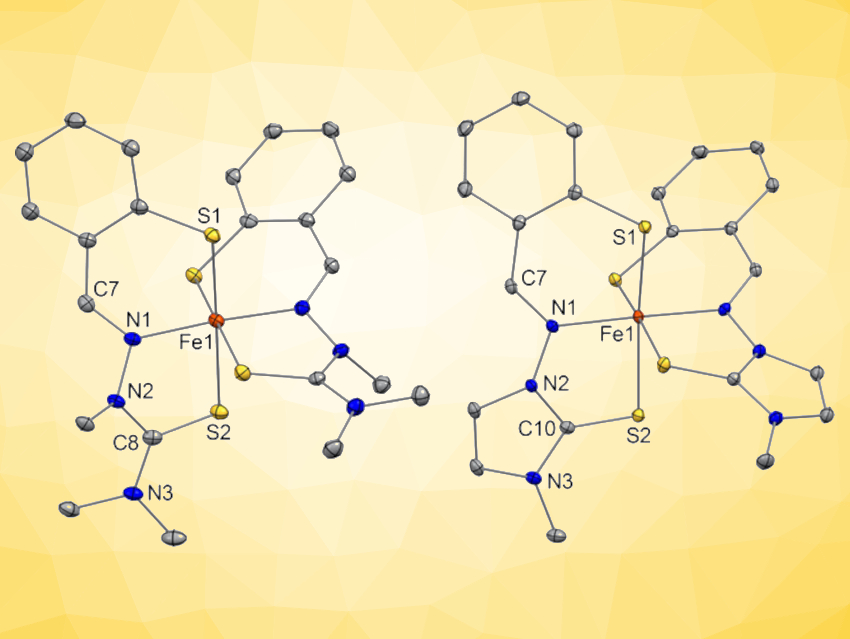
Elisa Tomat, The University of Arizona, Tucson, USA, and colleagues have synthesized disulfide-based prochelators featuring alkylated thiosemicarbazone and imidazole-2-thione binding units. The corresponding thiols form iron(III) complexes (pictured) in aerobic conditions, which was confirmed by crystallography and electron paramagnetic resonance (EPR) measurements.
The prochelators form stable disulfide conjugates with serum albumin, which is a major component of cell growth media and the most abundant protein in human blood. Mass spectrometry (MS) experiments showed clear covalent modification of the protein upon 24-hour incubations. Thus, serum albumin could serve as an effective drug-delivery carrier.
In a panel of breast, ovarian, and colorectal cancer cell lines, the conjugates showed antiproliferative activities at sub-micromolar concentrations. In contrast, normal lung cells are far less susceptible to the compounds, and the antiproliferative activities are up to two orders of magnitude lower. The new prochelators, therefore, combine a promising toxicity profile with albumin bioconjugation, which could provide an enhanced lifetime in blood circulation and tumor accumulation. Further investigations could provide data about the specific intracellular effects of the iron-binding systems as well as their anticancer activity in vivo.
- Albumin Conjugates of Thiosemicarbazone and Imidazole‐2‐thione Prochelators: Iron Coordination and Antiproliferative Activity,
Yu‐Shien Sung, Wangbin Wu, Megan A. Ewbank, Rachel D. Utterback, Michael T. Marty, Elisa Tomat,
ChemMedChem 2021.
https://doi.org/10.1002/cmdc.202100278