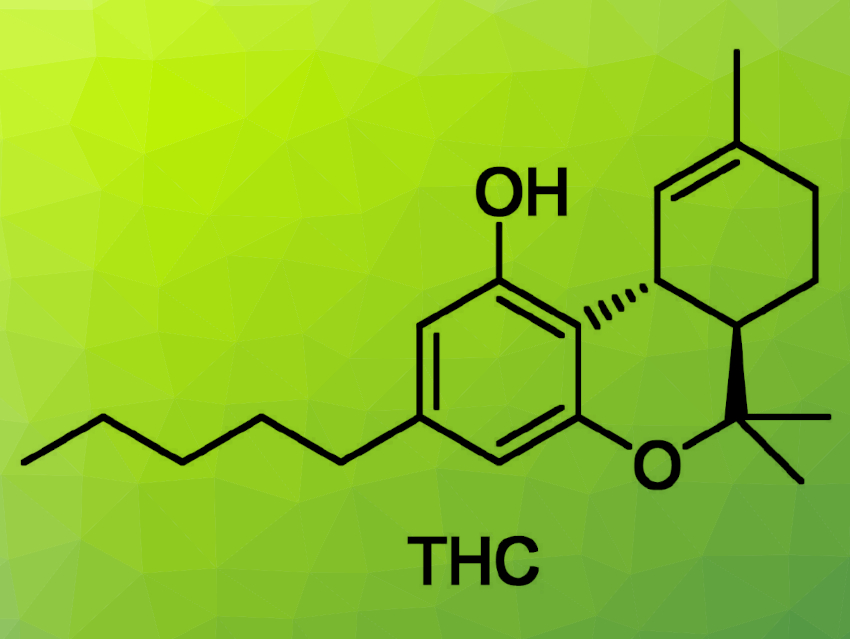
Cannabinoids are known for getting people high, but they also have immense pharmacological capabilities. Stefan Bräse, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have found a hub-based synthetic route to Δ9-tetrahydrocannabinol (THC, pictured above), the substance responsible for the main psychoactive effects associated with cannabis. Their approach centers around a Diels–Alder reaction, which is used to construct the stereochemically demanding three-carbon-ring scaffold of THC under mild conditions.
Cannabinoid Synthesis
Arguably, the most commonly used method worldwide for producing tetrahydrocannabinol is the extraction and purification from cannabis plants. However, the large-scale growth of THC-rich cannabis is strictly regulated or entirely forbidden in most countries. One semisynthetic approach is to isolate cannabidiol, which is not psychoactive, from less potent cannabis strains, and apply a ring-closing chemical step to form the three-ring chemical scaffold of THC.
Researchers have a huge interest in exploring the pharmacological effects of cannabinoid derivatives and are discovering derivatives with tailor-made, beneficial effects. Cannabidiol, with its two rings, for example, is not psychoactive and is investigated for its relaxing properties. Other three-ring relatives can be pharmacologically useful, too. They can have antiemetic, appetizing, and relaxing properties and can alleviate the side effects of chemotherapies. Some are used to treat nausea, loss of appetite, and spasms.
THC itself causes euphoria and changes in perception, but it is nowhere near the strongest cannabinoid derivative. A synthetic derivative called HU-210, with slightly different substituents on the three-ring scaffold, surpasses THC in its activity by a factor of 100–800.
Hub-Based Synthesis
In order to explore the effects of different substituents, researchers need efficient synthetic strategies to access the THC scaffold. The team focused on purely chemical synthetic methods. Their aim was to construct a “synthetic hub” from which many THC derivatives could be derived by similar synthesis protocols.
 This synthetic hub had already been identified: benzopyranes or chromenes supply the two basic THC rings, and if they are equipped with a vinyl group (example pictured on the right), the third ring could be attached via a Diels–Alder approach. The researchers knew this approach had been tried before by another group, but with mixed results [1].
This synthetic hub had already been identified: benzopyranes or chromenes supply the two basic THC rings, and if they are equipped with a vinyl group (example pictured on the right), the third ring could be attached via a Diels–Alder approach. The researchers knew this approach had been tried before by another group, but with mixed results [1].
The team discovered that maleic acid anhydride did a better Diels–Alder job: It successfully attached the third carbon ring to the chromene structure, yielding a three-ring product quantitatively in one step and with the correct stereochemistry. From there, the researchers synthesized the complete THC in five further steps. They state that this hub, the vinyl chromenes, could also be accessed by chemical synthesis from commercially available resorcinols.
Despite the successful synthesis, the steps from Diels–Alder adduct to complete THC gave low yields, and the conversions were elaborate. However, the team’s ultimate goal was THC synthesis as a proof-of-concept, and they posit that other products could be easier to access and could serve as new THC analogs for pharmaceutical research.
- The Diels–Alder Approach towards Cannabinoid Derivatives and Formal Synthesis of Tetrahydrocannabinol (THC),
Thomas Hurrle, Franziska Gläser, Manuel C. Bröhmer, Martin Nieger, and Stefan Bräse,
ChemistryOpen 2021, 10, 587–592.
https://doi.org/10.1002/open.202000343
Reference
- [1] 3-Vinylcoumarins and 3-vinylchromenes as dienes. Application to the synthesis of 3,4-fused coumarins and chromenes,
Toru Minami, Yasuyuki Matsumoto, Seigo Nakamura, Shinichiro Koyanagi, Masahiko Yamaguchi,
J. Org. Chem. 2002, 57, 167–173.
https://doi.org/10.1021/jo00027a032