Nitro fatty acids (NFAs, pictured on the right) are nitrated derivatives of unsaturated fatty acids. They are bioactive and can trigger, e.g., anti-inflammatory and cell-protective effects. The electrophilic nitroolefin unit makes NFAs reactive Michael acceptors. They can modify proteins via the nitroalkylation of nucleophilic amino-acid residues. NFAs are generally prepared by the reaction of the corresponding unsaturated fatty acids with reactive nitrogen species.
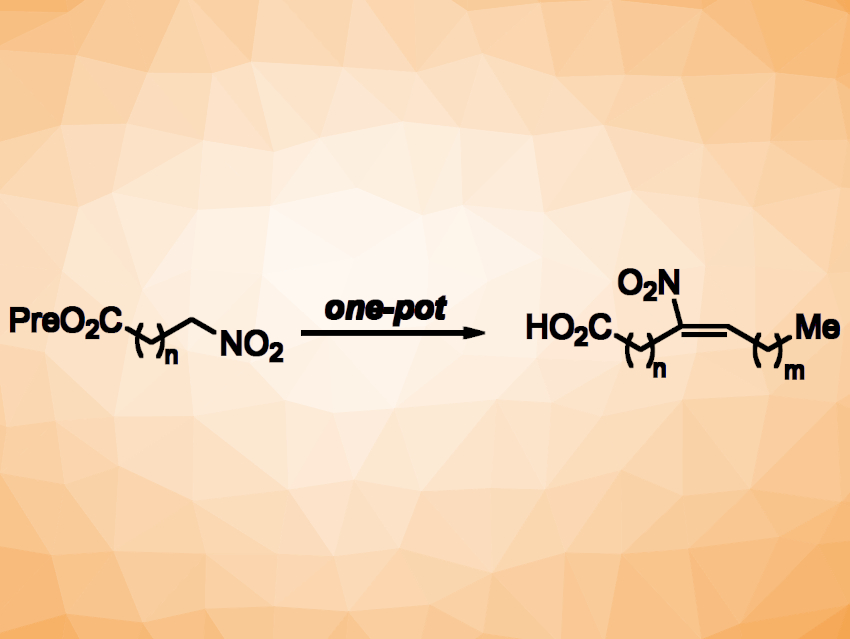
Georg Manolikakes, Technical University of Kaiserslautern, Germany, and colleagues have developed a streamlined process for the synthesis of NFAs. The team was able to prepare products with a large structural diversity using a modular approach. They started by protecting a carboxylic acid building block with a prenyl (Pre, 3-methylbut-2-en-1-yl) group. The resulting intermediate was reacted with NaNO2 to give a nitroalkane. The team then performed a Henry reaction with an aldehyde to form a C–C bond and create the fatty acid chain, followed by a dehydration of the resulting nitroalcohol to introduce the desired double bond. A final deprotection step then gave the desired NFA.
Three steps of the synthetic sequence—the Henry reaction, the dehydration, and the deprotection— were performed in a one-pot operation, which allowed the team to rapidly assemble NFAs. According to the researchers, this streamlined protocol could be a useful tool for the evaluation of the therapeutic potential of NFAs. Using the developed method, they prepared useful derivatives such as alkyne-labeled or deuterated NFA analogues.
- Streamlined One-Pot Synthesis of Nitro Fatty Acids,
Mohamed Hassan, Sara-Cathrin Krieg, Cedric Ndefo Nde, Jessica Roos, Thorsten J. Maier, Eman A. El Rady, Mohamed A. Raslan, Kamal U. Sadek, Georg Manolikakes,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100247



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)