Metal–organic cages are versatile molecular containers for the storage of molecules in a variety of applications, from catalysis to sensing. They are self-assembled from metal cations and organic ligands. However, the synthesis of these ligands is often a challenging and time-consuming multi-step process.
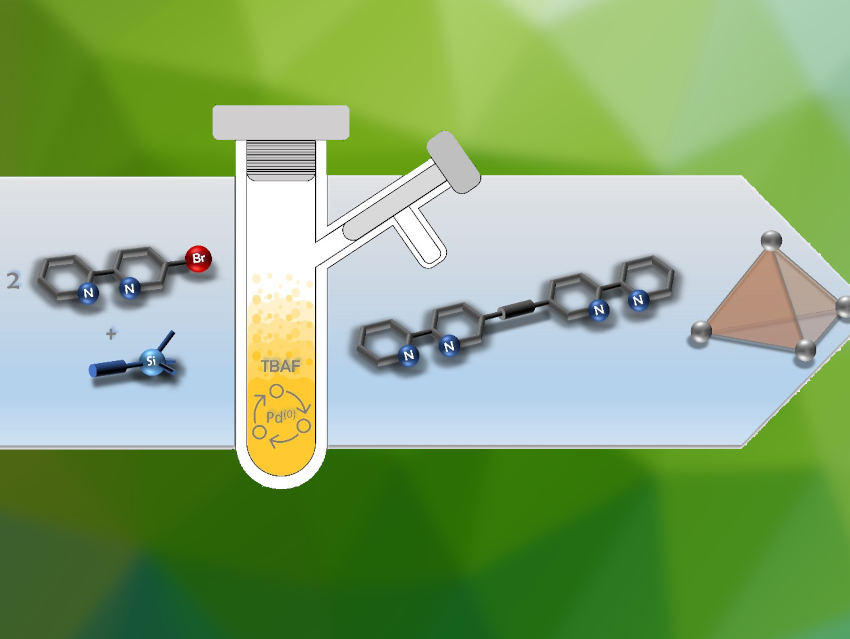
Anna J. McConnell, University of Kiel, Germany, and colleagues have developed a synthetic procedure for the efficient preparation of bipyridine- and benzimidazole-based ligands in the form of symmetric di(hetero)arylalkynes from simple (hetero)aryl halide precursors in one step (bipyridine-based example pictured below). The procedure uses cheap, commonly used reagents in a Sonogashira-type cross-coupling reaction. The team used Pd(PPh3)4 as the catalyst, trimethylsilylacetylene as an acetylene source, tetrabutylammonium fluoride (TBAF) as a base, activator, and deprotection reagent, and tetrahydrofuran (THF) as the solvent.
A variety of ligands were synthesized in good yields and with short reaction times. They can be used for the self-assembly of Co4L6-type metal-organic cages. Using this simple one-pot procedure, such ligands can be prepared with reduced synthetic effort, which could facilitate cage discovery. The researchers propose this procedure could not only be used for the synthesis of heterocyclic ligands but also for a wider scope of symmetric diarylalkynes.
- Copper‐Free One‐Pot Sonogashira‐Type Coupling for the Efficient Preparation of Symmetric Diarylalkyne Ligands for Metal‐Organic Cages,
Marc Lehr, Tobias Paschelke, Victoria Bendt, André Petersen, Lorenz Pietsch, Patrick Harders, Anna McConnell,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100275




![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)