Organometallic reagents are essential tools in synthetic chemistry. They work even better and more effectively in combination with alkali alkoxides. The exact nature of this effect has never been well understood. Eva Hevia, University of Bern, Switzerland, and colleagues have performed a detailed study of the mechanism of the reaction of aryl bromides with organo-magnesium reagents and lithium alkoxides. The team found that a complex equilibrium of bimetallic intermediates plays a key role.
Halogen/Metal Exchange
Substituted aromatic ring systems are an important class of building block for the synthesis of many products, including pharmaceuticals, agrochemicals, and natural substances. However, the required functional side groups cannot generally be hooked onto the ring systems in a simple way. A widely employed method uses a detour through a halogen/metal exchange. First, a halogen atom, such as bromine, is attached to the desired position on the ring system. With the use of special organometallic reagents—compounds with at least one metal–carbon bond—the Br atom can be exchanged for a metal atom, such as magnesium. The Mg atom can then easily be replaced by the desired substituent.
Interestingly, the use of alkali alkoxides in conjunction with the organometallic reagents causes synergistic effects; that is, increased reactivity and altered reactivity profiles. In this manner, lithium alkoxides (LiOR) activate the organo-magnesium reagent sBu2Mg (di-sec-butylmagnesium), allowing for an Mg/Br exchange with bromine-containing aromatics.
A New Type of Bimetallic Schlenk‐Type Equilibrium
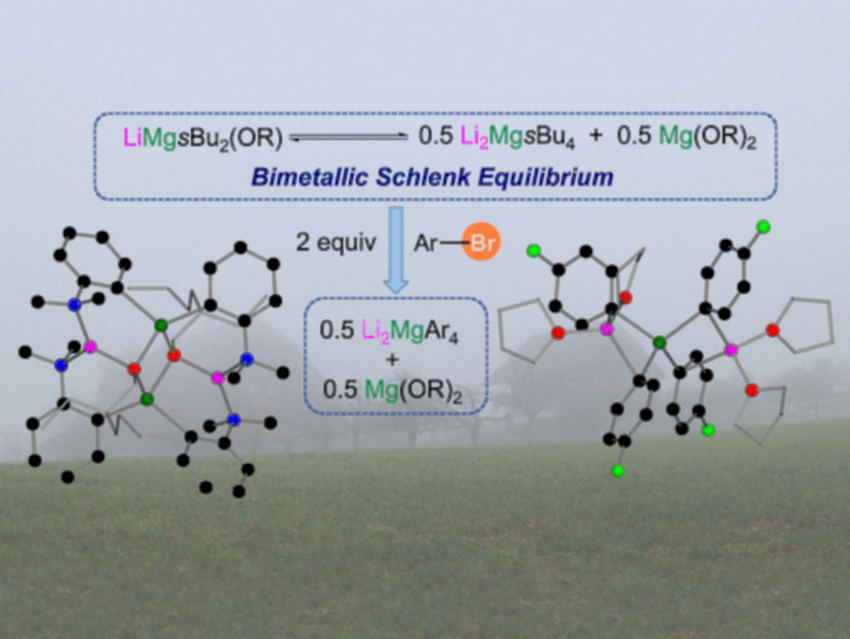
The researchers took a closer look at this type of reaction, using various methods to trap and analyze the organometallic intermediates that are formed. “We have found a complex equilibrium between various bimetallic species,” says Hevia. “The key components are two different intermediates of the Br/Mg exchange that depend on the substitution pattern of the aromatic substrate.”
Detailed NMR-spectroscopic studies showed that the heart of the reaction is a new version of the Schlenk equilibrium between bimetallic intermediates, lithium magnesiates, which have different sets of ligands and different Li:Mg ratios. Surprisingly, the in–situ generation of an alkyl-rich lithium magnesiate is revealed as the active species of the Mg/Br exchange.
“Our insights advance our understanding of the modus operandi of these fascinating bimetallic systems,” states Hevia, “which could pave new paths toward new exciting synthetic applications.”
- Untangling the Complexity of Mixed Lithium/Magnesium Alkyl/Alkoxy Combinations Utilised in Bromine/Magnesium Exchange Reactions,
Leonie J. Bole, Neil R. Judge, Eva Hevia,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202016422