Acenes are a class of polycyclic aromatic hydrocarbons (PAHs) and consist of linearly fused benzene rings. Larger acenes, especially those with more than six annulated benzene rings, are interesting compounds, both for fundamental studies and for their potential applications in organic electronics. However, the longer the acenes, the lower their chemical stability. Acene backbones can, for example, be stabilized by peri-annulation. Although these compounds are no longer “real” acenes, they have fascinating optoelectronic properties of their own. Until now, such peri-annulated acenes were based mainly on pyrene units (pictured below), which often break π-conjugation along the longitudinal acene axis.
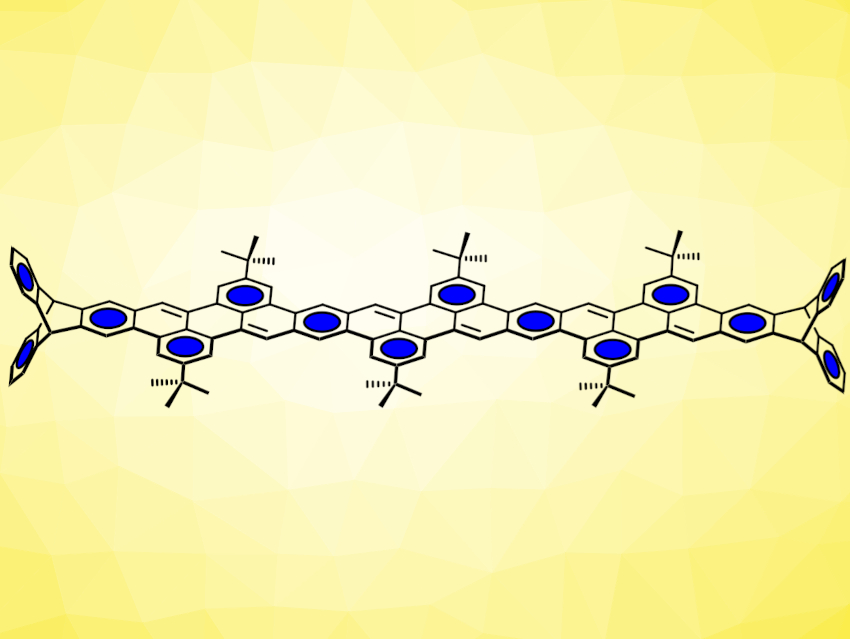
Michael Mastalerz, University of Heidelberg, Germany, and colleagues have introduced a new type of peri-annulated acenes based on perylene units (pictured below), which are built up in the final synthesis step. The team used Suzuki-Miyaura cross-coupling reactions between diboronic esters and bromoaldehydes to build aldehyde-functionalized, chain-like intermediates. These were then reacted with t-BuOK to induce condensation reactions, close the perylene ring systems, and give the desired products.

Using this strategy, the team synthesized a small series of conjugated oligomers with 5, 9, and 13 linearly annulated benzene rings (longest example pictured above). The compounds show distinct emission of light with high quantum yields, even for the longest one with 13 rings.
- Benzo‐fused Perylene Oligomers with up to 13 Linearly Annulated Rings,
Michael Mastalerz, Xuan Yang, Frank Rominger,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202017062