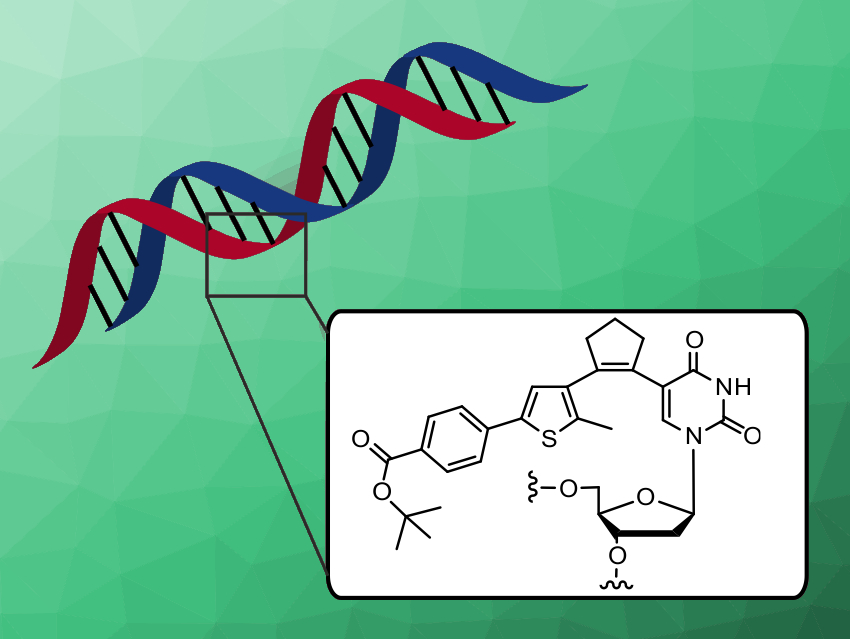
Many biological processes are controlled by light, which acts mostly on photoresponsive proteins. Nucleic acids, on the other hand, must be derivatized with photochromic components to enable reversible switching.
Andres Jäschke, Heidelberg University, Germany, and colleagues have developed pyrimidine nucleosides with the structural features of diarylethenes. Diarylethenes undergo electrocyclic ring closure upon irradiation with UV light which can be reversed by visible light. A library of deoxyuridine and deoxycytidine derivatives was synthesized using a sequence of borylation and Suzuki cross-coupling reactions. This is the first systematic structure–activity study of pyrimidine nucleoside‐based diarylethenes photoswitches.
Two compounds with near-quantitative, fully reversible back-and-forth switching, high quantum yields, and high thermal and photochemical stability were identified. These two candidates were incorporated by solid-phase synthesis into DNA carrying the sequence of a promoter for an RNA polymerase enzyme. The DNA turned out to be photoresponsive and allowed to modulate the rate of enzymatic RNA synthesis by light.
According to the researchers, this reversible control of DNA structure and function by light offers promising applications in synthetic biology, bionanotechnology, and next-generation computing.
- Development of High‐Performance Pyrimidine Nucleoside and Oligonucleotide Diarylethene Photoswitches,
Theresa Kolmar, Simon M. Büllmann, Christopher Sarter, Katharina Höfer, Andres Jäschke,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202014878