Allylation reactions are useful in organic synthesis. Allylations can be performed, e.g., under Barbier conditions using redox-active metals (zinc, indium, etc.) in low oxidation states via an in situ reaction with allyl halides in the presence of reactive electrophiles. This requires a stoichiometric amount of metal or another stoichiometric sacrificial reducing agent.
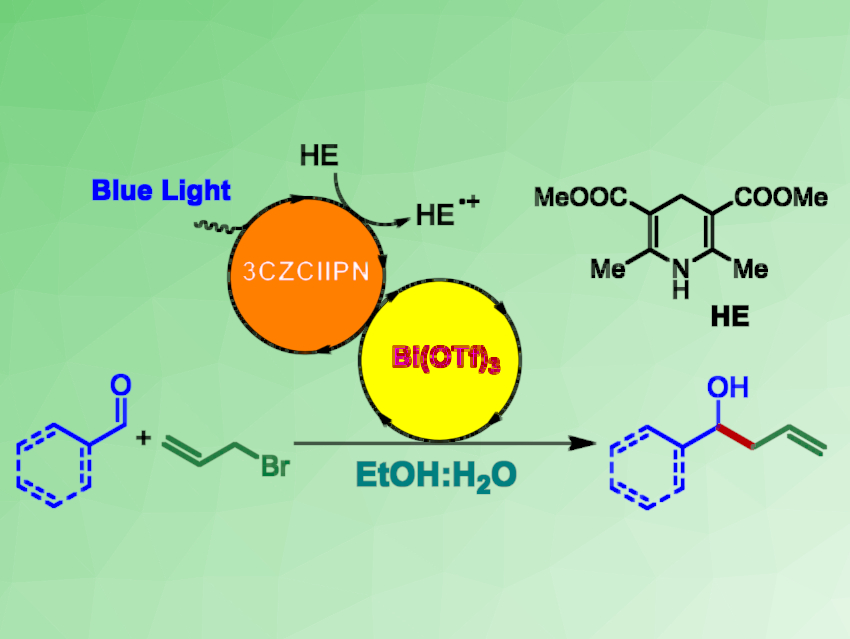
Andrea Gualandi, Pier Giorgio Cozzi, Università di Bologna, Italy, and colleagues have developed a photoredox Barbier allylation of aldehydes (pictured) that uses catalytic amounts of non-toxic bismuth (Bi(OTf)3) in the presence of an organic dye (3CzClIPN) as a photocatalyst and a Hantzsch ester (HE) as a sacrificial reducing agent. Under photoredox conditions using blue LED lights, reactive allylbismuth intermediates are generated from allylbromide. These intermediates can then react with the aldehyde to give the desired homoallylic alcohols as products.
The reaction has a broad scope, can be carried out in EtOH/water under mild conditions, use low-toxicity bismuth, tolerates oxygen, and does not require other metals as co‐reductants. These properties make the reaction promising for the green and sustainable synthesis of homoallylic alcohols.
- Photoredox Allylation Reactions Mediated by Bismuth in Aqueous Conditions.,
Simone Potenti, Andrea Gualandi, Alessio Puggioli, Andrea Fermi, Giacomo Bergamini, Pier Giorgio Cozzi,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202001640