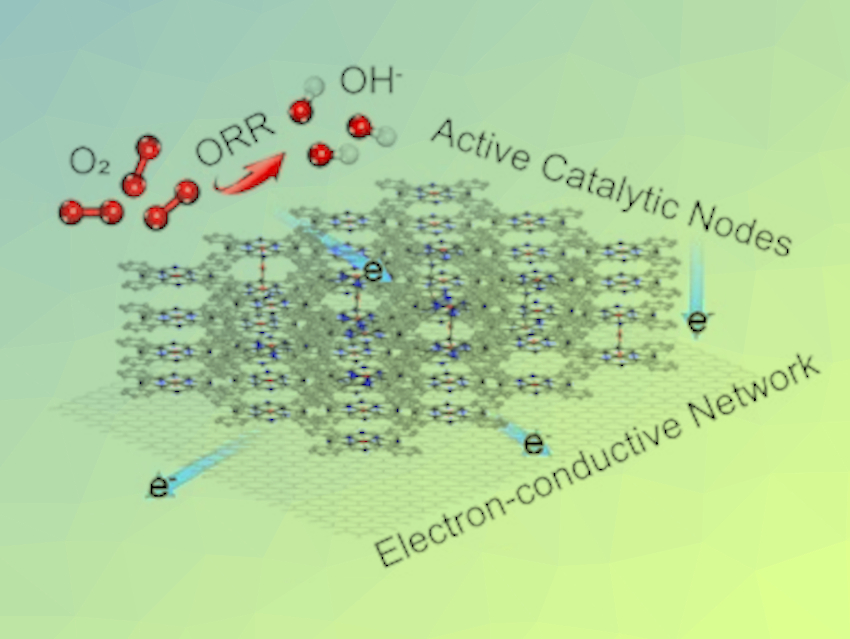
The electrocatalytic oxygen reduction reaction (ORR) is important for renewable energy conversion systems, such as fuel cells and metal–air batteries. Single‐atom catalysts (SACs), where many atomic‐metal sites are separated from each other on solid supports, could be useful for ORR processes. Unfortunately, traditional SACs, made by embedding metal−nitrogen coordination sites in a zero- or one‐dimensional (0D/1D) carbon matrix, suffer from a lack of stability and electronic conductivity.
Zigeng Liu, Institut für Energie und Klimaforschung, Forschungszentrum Jülich GmbH, Jülich, Germany, Wei Wang, Tianjin University, China, and colleagues have developed SACs with improved properties. The team used a composite structure of 1D Fe SACs conjugated on a 2D graphene film. The catalyst was synthesized by pyrolyzing iron phthalocyanine under mild conditions and then transferring the generated species using inert gas to a lower-temperature region to form the final Fe SAC layers on the graphene substrate (pictured).

The product shows electrochemical ORR efficiency and stability compared with commercial Pt/C catalysts in alkaline solution. Based on density functional theory (DFT) calculations, the team found that the intrinsic activity of the catalyst originated from a newly formed FeN4-O-FeN4 bridge structure with an optimized adsorption ability towards ORR intermediates.
- Reconstructing 1D Fe Single‐atom Catalytic Structure on 2D Graphene Film for High‐Efficiency Oxygen Reduction Reaction,
Guangqi Zhu, Yanling Qi, Fan Liu, Shenqian Ma, Guolei Xiang, Fengmin Jin, Zigeng Liu, Wei Wang,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202002359