Helicenes are screw-shaped chiral molecules that show high differential emission of right- and left-handed circularly polarized (CP) light. These compounds may be useful for CP organic light-emitting diodes (OLEDs). However, the fluorescence intensity of helicenes consisting of six-membered rings is limited by intersystem crossing.
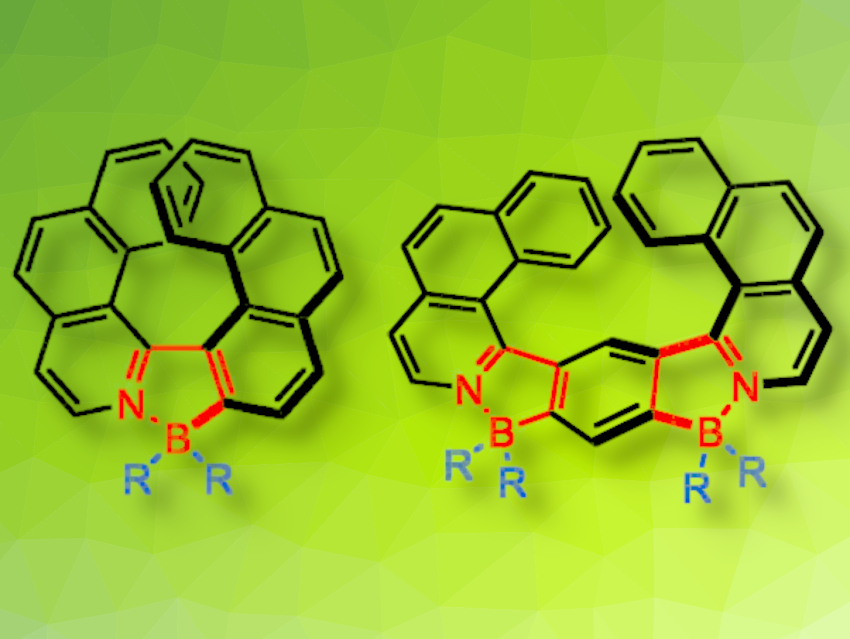
Agnieszka Nowak-Król, University of Würzburg, Germany, and colleagues have synthesized and characterized two types of helicenes incorporating five-membered azaborole rings (pictured below). The molecules were prepared via a modular approach from achiral building blocks. First, flexible biaryl and triaryl precursors were prepared via cross‐coupling reactions. Then, boron atoms were introduced via a reaction with BBr3 in the presence of iPr2NEt.

The products show a high differential absorption of CP light and strong fluorescence with high fluorescence quantum yields. High quantum yields are retained in the solid state, which is attributed to the non-planar geometry of helicenes. According to the researchers, helically chiral boron compounds could be useful, e.g., as emitters for OLEDs or fluorescent probes for bioimaging.
- Modular Synthesis of Organoboron Helically Chiral Compounds: Cutouts from Extended Helices,
Agnieszka Nowak-Król, Julian Full, Santosh P. Panchal, Julian Götz, Ana-Maria Krause,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202014138


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
