Water electrolysis driven by sustainable electricity can be used to produce green hydrogen fuel. However, the oxygen evolution reaction (OER) is a bottleneck of water electrolysis. This reaction could benefit from the development of electrocatalysts with improved effectiveness.
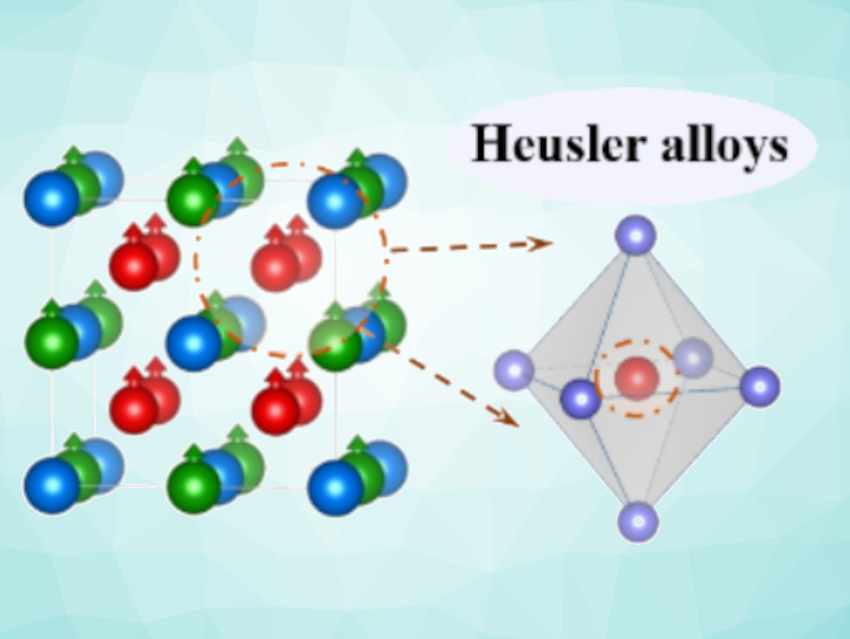
Claudia Felser, Max Planck Institute for Chemical Physics of Solids, Dresden, Germany, Harun Tüysüz, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have used Co2YZ-type Heusler compounds as OER catalysts. Heusler compounds are intermetallic compounds with the chemical formula X2YZ or XYZ, where X and Y are transition metals and Z is usually a main group element or a transition metal. The team prepared a range of Co2YZ-type compounds (Co2MnZ with Z = Ti, Al, V, Ga; Co2VZ with Z = Sn, Ga) by arc melting stoichiometric amounts of the respective elements.
These alloys have high conductivities and crystallinities. They also have well-defined topological surfaces that are suitable for electrocatalytic applications. The electronic structure of reactive cobalt sites was adjusted by varying the other, unreactive metal sites. A systematic electrocatalytic investigation showed a correlation between the occupancy of d-type orbitals of the cobalt centers and OER performance, with Co2MnTi and Co2VSn achieving the best OER activities.
- Tunable eg orbital occupancy in Heusler compounds for oxygen evolution reaction,
Mingquan Yu, Guowei Li, Chenguang Fu, Enke Liu, Kaustuv Manna, Eko Budiyanto, Qun Yang, Claudia Felser, Harun Tüysüz,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202013610