The use of chiral Brønsted acids has led to a variety of new enantioselective transformations. However, the generation of vinylic carbocations from alkynes and their use in asymmetric catalysis is not well understood so far. This may be explained by the weak basicity of unactivated alkynes, which hampers their protonation with chiral Brønsted acids, and by the lack of interaction between the conjugate base of the Brønsted acid and the carbocation, which often results in weak chiral inductions.
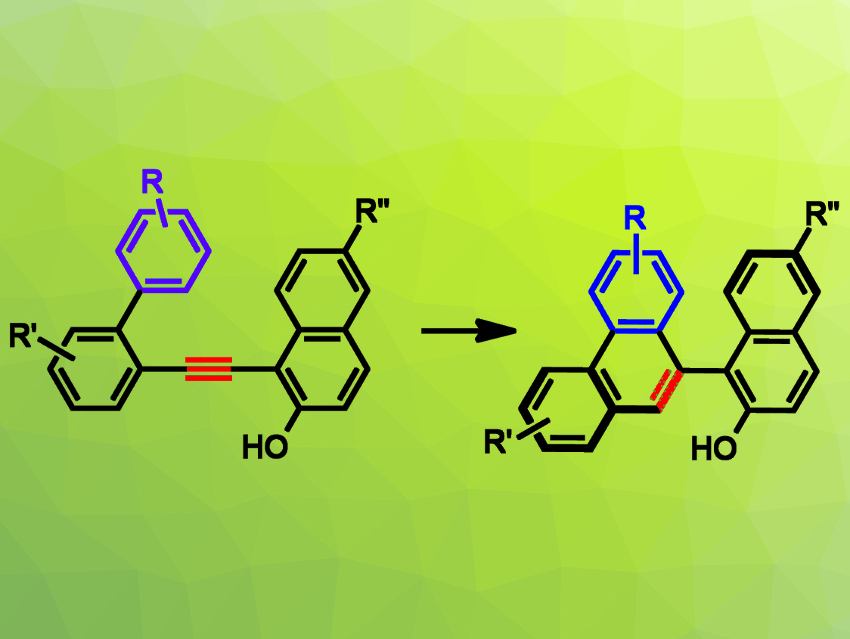
Patrick Y. Toullec, University of Bordeaux, France, and colleagues have developed an asymmetric Brønsted-acid-catalyzed intramolecular hydroarylation of alkynes (pictured). The team used chiral N-triflyl phosphoramides, in combination with a 2-naphthol substituent on the alkyne, to allow the formation of atropisomeric phenanthrenes (pictured on the right) under mild, metal‐free reaction conditions.
The desired chiral atropisomeric phenanthrenyl-naphthols were obtained in high yields (up to 99 %) and good enantioselectivities (up to 96:4 e.r.). According to the researchers, this is the first example of an enantioselective carbocyclization of an alkyne‐containing substrate catalyzed by chiral Brønsted acids. They found that the carbenic nature of the stabilized vinyl cation intermediate can be used to initiate an alkyne/alkane cycloisomerization reaction.
- Brønsted Acid‐Catalyzed Enantioselective Cycloisomerization of Arylalkynes,
Julien Gicquiaud, Baptiste Abadie, Kalyan Dhara, Murielle Berlande, Philippe Hermange, Jean‐Marc Sotiropoulos, Patrick Y. Toullec,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202003783