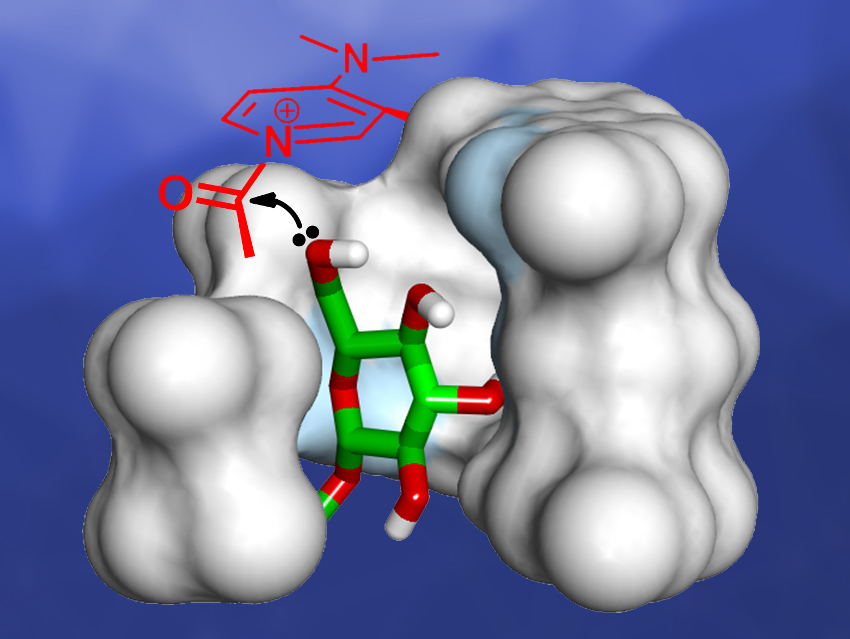
The regioselective acylation of carbohydrates and polyols without repeated protection and deprotection steps is an attractive research target in synthetic chemistry. Foldamers, i.e., chain-like molecules that fold into a conformationally ordered state in solution, could be used to catalyze this type of reaction in a site-selective manner. Numerous aromatic foldamers having folding‐generated internal cavities have been developed and can act as synthetic receptors capable of binding ions and molecules. The properties of the internal cavities can be modified to achieve selective binding of target guests.
Geunmoo Song and Kyu‐Sung Jeong, Yonsei University, Seoul, Republic of Korea, have developed indolocarbazole-naphthyridine oligomers that contain a 4-(N,N-dimethylamino)pyridine (DMAP) moiety at one end of the strand. DMAP is a well‐known nucleophilic catalyst for the acylation of alcohols and amines. The oligomers fold into helical conformations and generate an internal binding site with a closely located active DMAP site (pictured)—similar to the active site of an enzyme.
The helical foldamers can catalyze the acetylation of octyl β-D-glucopyranoside with high regioselectivity up to 90 % for the 6-O-acetylated product. According to the researchers, this kind of foldamer catalyst could be useful as a biomimetic model for enzymatic reactions, while modifications are needed to develop more practical catalysts for the transformation of polyols and carbohydrates with high regioselectivity.
- Aromatic Helical Foldamers as Nucleophilic Catalysts for the Regioselective Acetylation of Octyl β‐D‐Glucopyranoside,
Geunmoo Song, Kyu‐Sung Jeong,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000685