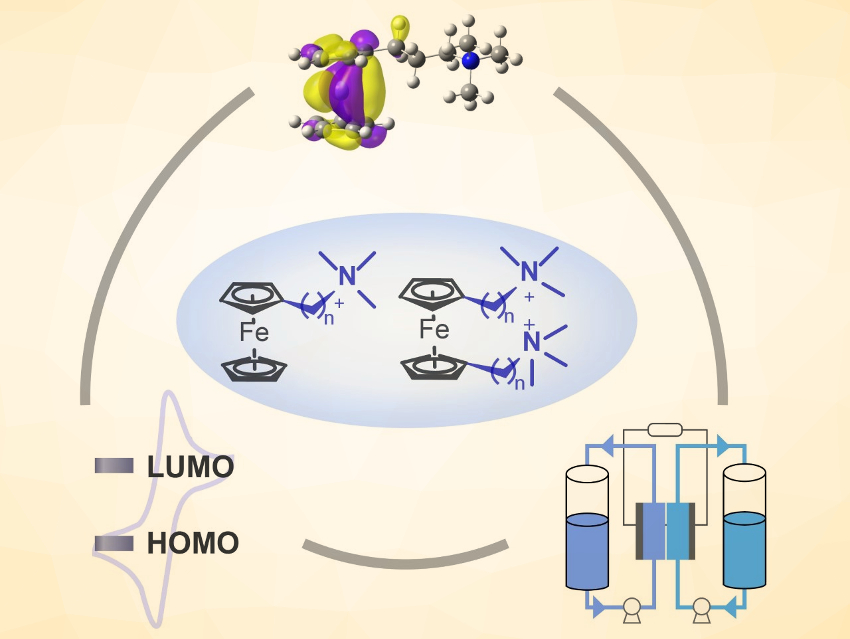
The large-scale storage of renewable electricity from fluctuating energy sources is important for the wide adoption of these environmentally friendly technologies. Aqueous organic flow batteries (AOFBs) use electroactive water-soluble organic molecules for safe and cost-effective energy storage, and thus, could potentially solve this problem. However, so far AOFB development is hindered by the limited choice of redox-active organics to be used as the catholyte. In addition, how the catholyte structures influence the battery lifespan remains largely unexplored.
Zhengjin Yang, Tongwen Xu, University of Science and Technology of China, Hefei, and colleagues have designed a series of six ferrocene catholytes and investigated how the catholyte structures, electrochemical properties, and cycling stability correlate with each other. The six water‐soluble ferrocene derivatives (general structures pictured above) are 3‐trimethylammonio)propyl)‐ferrocene bromide, 4‐trimethylammonio)butyl)‐ferrocene bromide, (6‐trimethylammonio)hexyl)‐ferrocene bromide, bis((3‐trimethylammonio)propyl)‐ferrocene dibromide, bis((4‐trimethylammonio)butyl)‐ferrocene dibromide, and bis((6‐trimethylammonio)hexyl)‐ferrocene dibromide (BQH−Fc).
The team identified BQH-Fc as the candidate with the highest water solubility and the best stability. When paired with BTMAP-Vi, stable anolyte, no capacity loss was observed for a flow cell at 0.1 M, and a low capacity fade rate of 0.007 % h–1 was recorded at a high concentration of 1.5 M. The work could provide useful guidelines for the design of robust organic catholytes.
- Designer Ferrocene Catholyte for Aqueous Organic Flow Batteries,
Qianru Chen, Yuanyuan Li, Yahua Liu, Pan Sun, Zhengjin Yang, Tongwen Xu,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202002467