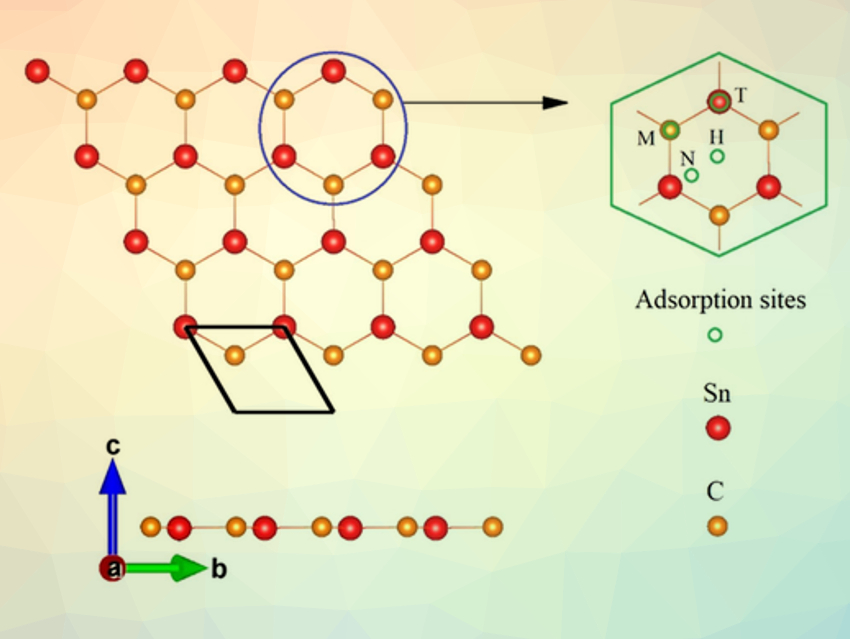
Metallic tin is a promising anode material for metal-ion batteries, such as potassium-ion batteries. However, Sn has a very low cycling performance. This is due to abrupt volume changes during the intercalation of potassium ions. The resulting cracks in the Sn anode can break the pathways between the current collector and Sn anode, and thus, lead to capacity decay.
Xiaofeng Fan, W. T. Zheng, Jilin University, Changchun, China, and colleagues have used theoretical calculations to study the structural, electronic, and electrochemical features of layered SnC, a 2D Sn-based material, and determine its suitability as an anode material. Layered SnC consists of a single atomic layer of Sn and C, packed in a honeycomb-like structure (pictured), similar to graphene. The team found that based on the calculated energies, SnC has a preferential interaction with K in an electrochemical environment. The layered structure is expected to prevent abrupt volume changes due to the insertion of K+ ions during charging/discharging. This effect is similar to layered carbon, such as graphite, which is used frequently in commercial Li-ion batteries.
Pristine layered SnC is a semiconductor. With the loading of K+ ions, it acquires metallic conductivity. Potassium can be stored on the lattice of a SnC monolayer with a high theoretical capacity of 410 mAh/g. The average open-circuit voltage is about 0.415 V. Potassium ions can rapidly migrate on the lattice of SnC sheet with a diffusion barrier of 0.17 eV. The good electronic conductivity and the high migration rate of K on an SnC sheet could lead to fast charge/discharge cycles. According to the team, monolayer SnC could be a useful anode material for next-generation potassium-ion batteries.
- Adsorption and Diffusion of Potassium on 2D SnC Sheets for Potential High-Performance Anodic Applications of Potassium-Ion Batteries,
Javed Rehman, Xiaofeng Fan, Amel Laref, W. T. Zheng,
ChemElectroChem 2020.
https://doi.org/10.1002/celc.202001039