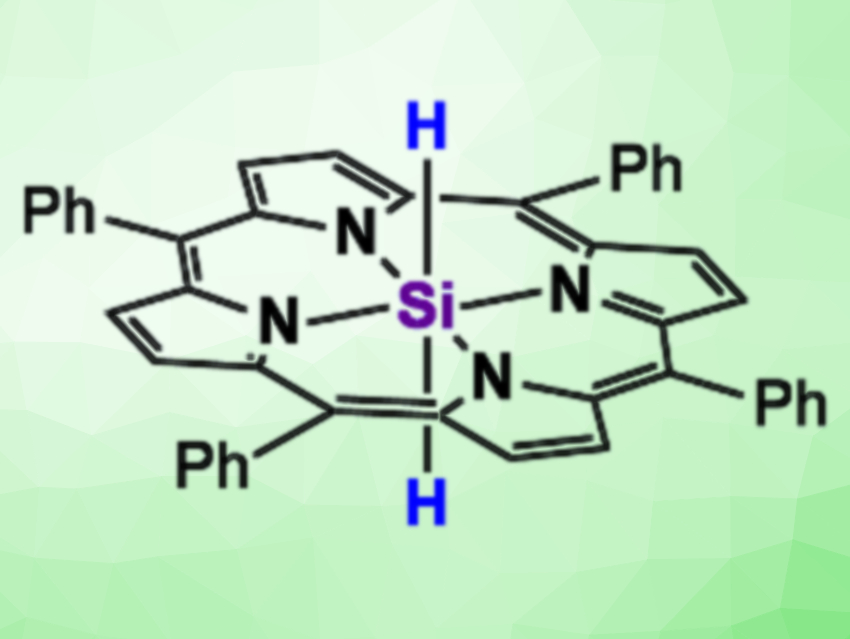
The catalytic hydrosilylation of CO2 is a method for the fixation of the greenhouse gas that provides value-added chemicals. Examples of catalyst-free methods for the hydrosilylation of CO2 under mild conditions, however, are rare.
Shintaro Ishida, Takeaki Iwamoto, Tohoku University, Sensai, Japan, Soichiro Kyushin, Gunma University, Kiryu, Japan, and colleagues have synthesized 5,10,15,20-tetraphenylporphyrinato(dihydrido)silicon(IV), a six-coordinate silicon dihydride (pictured above). The compound was synthesized via the reaction of a dichloro precursor with LiAlH4 in tetrahydrofuran (THF), followed by a brief exposure of the resulting solution to dry O2. The team found that the simple, previously unknown silicon dihydride undergoes a catalyst-free hydrosilylation with CO2 at ambient temperature and pressure. Combining this hydrosilylation with a subsequent hydrolysis (pictured below) gives formic acid from CO2.
![]()
The team performed density functional theory (DFT) calculations, which indicate that the hydride-donor ability of the six-coordinate silicon dihydride is exceptionally high for a neutral silicon hydride, and that the hydride transfer from silicon to CO2 is a key step for the non-catalytic hydrosilylation.
- A Six‐Coordinate Silicon Dihydride Embedded in a Porphyrin: Enhanced Hydride‐Donor Properties and the Catalyst‐Free Hydrosilylation of CO2,
Shintaro Ishida, Takuroh Hatakeyama, Takuya Nomura, Maiko Matsumoto, Kimio Yoshimura, Soichiro Kyushin, Takeaki Iwamoto,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202002587