Organic compounds with large systems of delocalized π-electrons can harvest light in the near-infrared (NIR) region of the electromagnetic spectrum and use it for energy conversion, charge transport, or re-emission of light (i.e., luminescence). The size of the π-system correlates with the red-shift of its main absorption, but molecules with large π-systems are often challenging and tedious to synthesize.
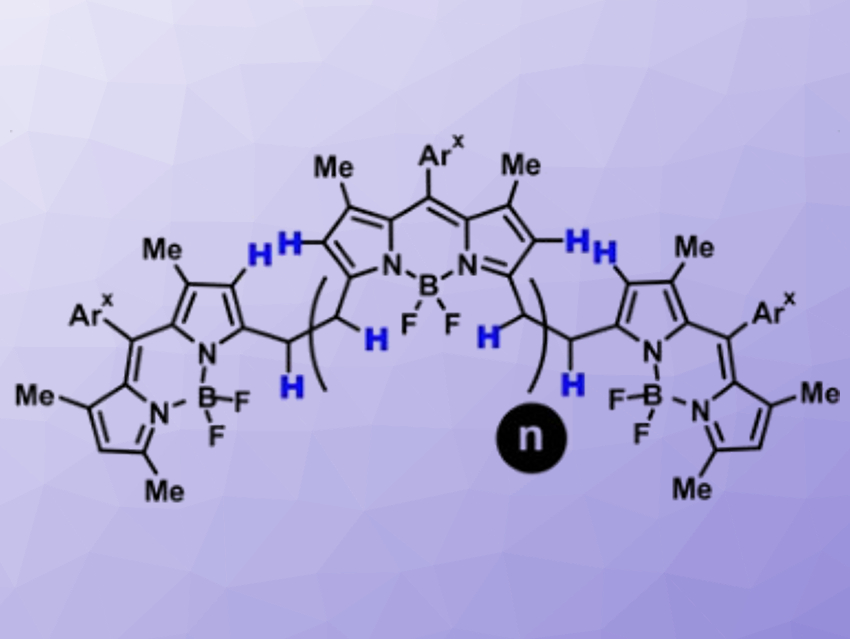
Daniel B. Werz, Technische Universität Braunschweig, Germany, and colleagues have developed a straightforward procedure to link traditional BODIPY-based fluorophores into fully conjugated oligomers. The team started from bridged BODIPY precursors and used an oxidative process to remove four hydrogen atoms from the bridge and form a connecting benzene ring. They used cheap FeCl3 as an oxidant and obtained up to octameric species with 31 linearly annulated rings (pictured below). These products strongly absorb NIR light at ca. 950 nm.

Interestingly, the number of integrated BODIPY units is reflected in the number of charges the molecule can take up. This suggests that the new oligo-BODIPYs might not only function as light-absorbing tools, but also as platforms for efficient charge transport, which could be useful for optoelectronic devices such as organic solar cells.
- Extended Benzene‐Fused Oligo‐BODIPYs: In Three Steps to a Series of Large, Arc‐Shaped, Near‐Infrared Dyes,
Atanu Patra, Lukas J. Patalag, Peter G. Jones, Daniel B. Werz,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202012335