Functionalized heterocycles are versatile building blocks in organic synthesis and useful structural motifs in drug discovery. Synthetic strategies for the incorporation of heteroaromatic scaffolds into potentially biologically active molecules are, thus, useful.
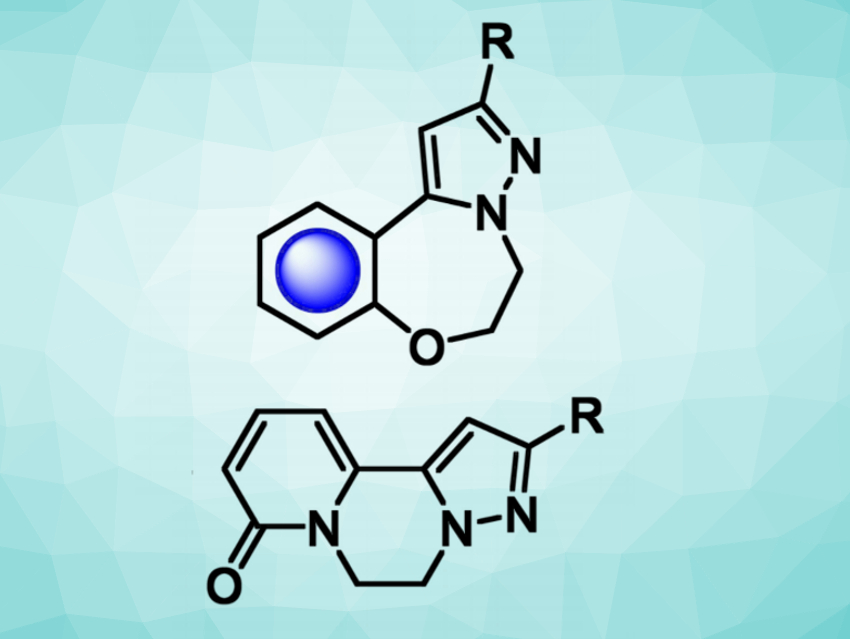
Joseph P. A. Harrity, University of Sheffield, UK, and colleagues have synthesized two libraries of 5-7-6- and 5-6-6-fused tricyclic compounds (structures pictured) using automated library synthesis. The tricyclic compounds were prepared from potassium 2-(3-phenyl-5-(trifluoroboranyl)-1H-pyrazol-1-yl)ethan-1-ol, an N-hydroxyethyl pyrazole trifluoroborate.
Suzuki-Miyaura reactions were used to couple this pyrazole-based substrate with fluoride-substituted arenes, followed by a cyclization to give 5-7-6-fused oxazepane-bridged tricycles (pictured at the top). The coupling reactions were performed using an automated library synthesis/purification system, which allows the efficient synthesis of many analogous compounds. 5,6,6-Fused piperazine-bridged tricycles (pictured at the bottom) were prepared via a coupling of the N-hydroxyethyl pyrazole trifluoroborate with 2-bromo-6-alkoxyazine derivatives, followed by hydrolysis and cyclization.
The obtained pyrazolotricycles have a wide range of physicochemical and pharmacokinetic properties. The automated synthesis method could be useful for the development of lead compounds in drug discovery studies.
- Design and Synthesis of New Pyrazole‐Based Heterotricycles and their Derivatization by Automated Library Synthesis,
Prisca Fricero, Laurent Bialy, Werngard Czechtizky, María Méndez, Joseph P. A. Harrity,
ChemMedChem 2020.
https://doi.org/10.1002/cmdc.202000187