Thioaldehydes are highly reactive species that are difficult to handle due to their strong tendency to polymerize. However, if they are reacted directly after in-situ generation, thioaldehydes can be effectively converted into 2H-thiopyrans via thia-Diels–Alder reactions. These sulfur heterocycles have applications in natural product synthesis and medicinal chemistry.
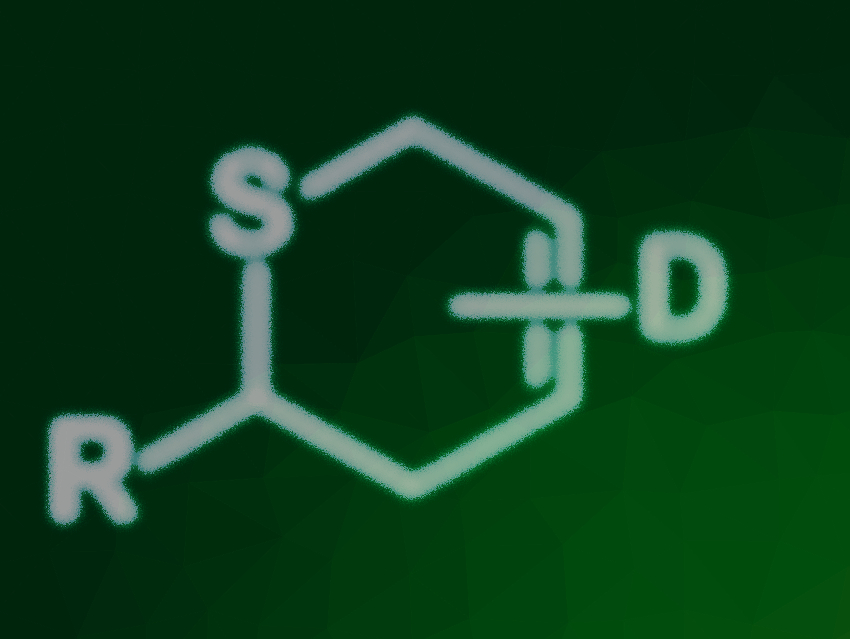
Florian Sachse and Christoph Schneider, University of Leipzig, Germany, have developed a process for the photochemical generation of thioaldehydes in a continuous flow process, which were reacted in situ with electron-rich 1,3-butadienes in thia-Diels–Alder reactions (pictured below, D = electron-donating group). The approach uses the known photochemical generation of thioaldehydes from phenacyl sulfides via a Norrish II reaction under UV light. The phenacyl sulfides were synthesized via reactions of α-bromo-acetophenone with the corresponding thiol, or via reactions of the thiol 2-mercapto-1-phenylethan-1-one with the corresponding bromides. Using these precursors and electron-rich 1,3-butadienes, a broad range of cycloadducts was obtained in good yields, short reaction times, and up to gram amounts.

The established process is very robust and allows for the scalable and high-yielding synthesis of 2H-thiopyrans across a broad substrate range. In addition, the process performs significantly better than the corresponding batch reaction in terms of productivity and yield.
- Continuous Flow Synthesis of 2H‑Thiopyrans via thia‐Diels‐Alder Reactions of Photochemically Generated Thioaldehydes,
Florian Sachse, Konrad Gebauer, Christoph Schneider,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202001343