Polypropylene (PP) is one of the most widely used plastics in the world. By controlling the spatial orientation of the propylene building blocks and additional polar components, it should be possible to create a new generation of attractive, engineered specialty plastics with improved wettability or enhanced degradability, based on PP. Kyoko Nozaki, The University of Tokyo, Japan, and colleagues have introduced the basis for a new class of palladium catalysts for such polymerizations.
Tacticity of Polypropylenes
The properties of PP depend largely on the spatial orientation of the individual monomers as they are added to the growing chain (tacticity). In atactic PP (aPP), the orientation is random. In syndiotactic PP (sPP), the CH3 side groups on the monomer alternately point toward the two sides of the polymer backbone. The most sought-after version—isotactic PP (iPP), in which all of the side groups point the same way—has particularly advantageous mechanical properties. The incorporation of additional functional, polar monomers into iPP is an important step toward the development of new plastics.
This type of copolymerization is heavily restricted with conventional Ziegler–Natta and metallocene catalysts because typical polar monomers first need to be “masked”. This means they must be attached to special protective groups. With nickel and palladium catalysts, it is possible to achieve this unmasked, but with significant losses in isotacticity. There has been some success with special nickel and palladium phosphine complexes, though the synthesis of these catalysts is arduous and time-consuming.
Efficient Synthetic Method for Ligand Development
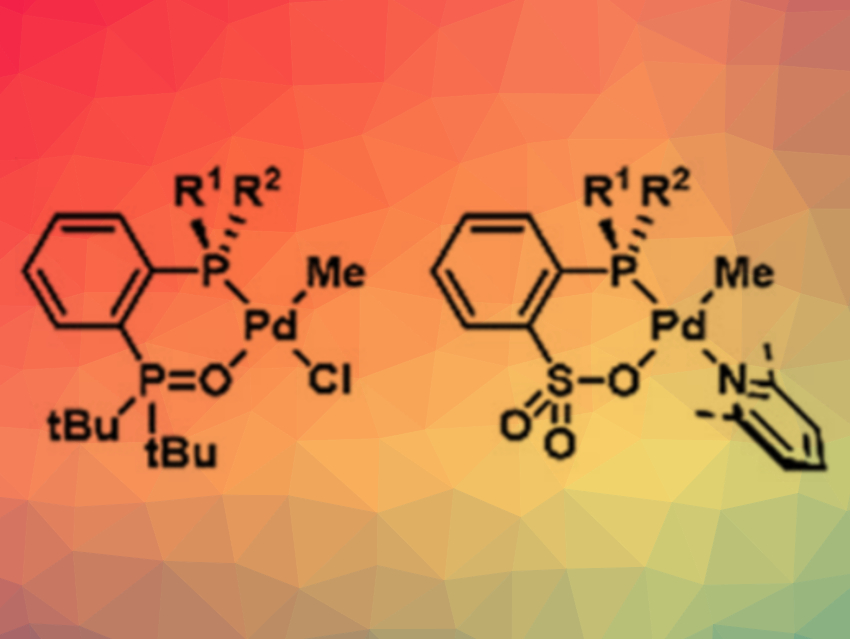
The team developed a new approach that allows more suitable catalysts to be produced much more easily. The spatial orientation of propylene monomers during polymerization is influenced by the special spatial structure (stereogenicity) at certain carbon atoms in the organic menthol substituents on the phosphine. The researchers wanted to develop phosphine compounds that have the required stereogenicity at the phosphorus atom.
To avoid the tedious synthetic challenges faced to date, the researchers developed significantly faster synthetic protocols using storable, modular building blocks, i.e., phosphinites, in place of phosphines. This allowed for the rapid and easy synthesis of many different phosphines and their corresponding palladium complexes. A rapid screening process successfully yielded suitable catalyst candidates.
In this way, the scientists found catalysts that polymerize propylene with polar monomers to form copolymers with particularly high isotacticity—a material they call isotactic polar polypropylene (iPPP).
- Expedient Synthetic Identification of a P‐Stereogenic Ligand Motif for the Palladium‐Catalyzed Preparation of Isotactic Polar Polypropylenes,
Falk William Seidel, Izumi Tomizawa, Kyoko Nozaki,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202009027