Nitrogenases are enzymes that can reductively fix atmospheric nitrogen into a bioavailable form. A less-noticed feature of these enzymes is their ability to convert carbon monoxide (CO) into hydrocarbons—a biological analogy to the Fischer–Tropsch process, in which CO and hydrogen are converted into liquid hydrocarbons industrially. However, among the three known classes of nitrogenases, only the vanadium (V)-dependent enzyme excels at performing this reaction.
Oliver Einsle and colleagues, University of Freiburg, Freiburg im Breisgau, Germany, have isolated vanadium nitrogenase from its native host, Azotobacter vinelandii. The team obtained the most highly resolved structure of the enzyme to date at 1.0 Å resolution using X-ray crystallography. They also performed turnover assays with carbon monoxide and obtained high-resolution structural data of the CO-bound state.
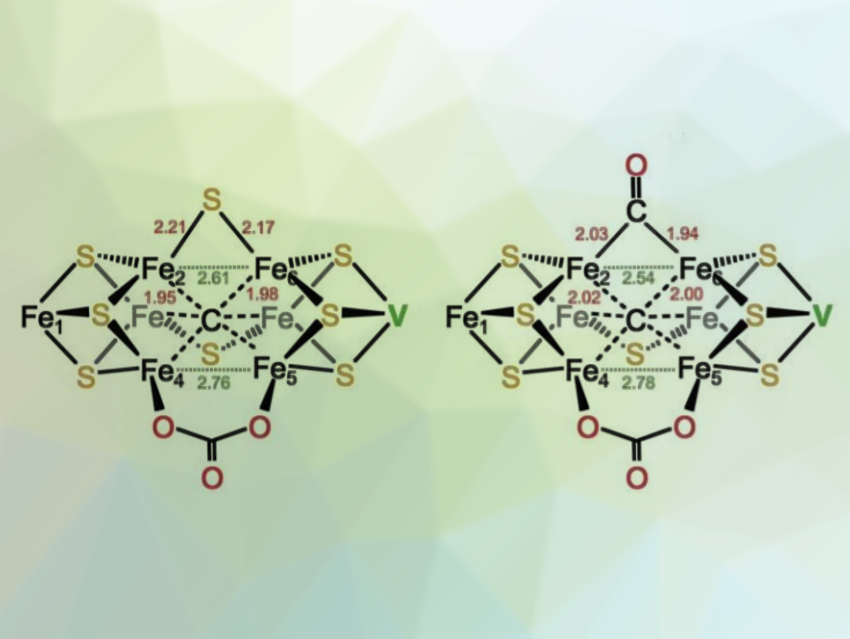
The researchers observed a remarkable rearrangement of the active-site cluster upon CO binding. The new CO ligand displaced a bridging sulfide (pictured), forming a bridging carbonyl adduct. Upon removal of CO, a light atom (likely an OH− group) can take its place at the cluster. According to the team, the work can provide valuable insight into the mechanism of action of V-nitrogenase in the fixation of both nitrogen and carbon.
- CO Binding to the FeV Cofactor of CO-Reducing Vanadium Nitrogenase at Atomic Resolution,
Michael Rohde, Katharina Grunau, Oliver Einsle,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202010790