Changing materials from an amorphous state to an ordered, crystalline structure is an interesting research target and can be useful for the development of functional materials. Ionic liquids are promising as safe electrolytes, e.g., for fuel cells. However, leakage risks and a sudden decrease in conductivity at low temperatures limit their application. These issues can be tackled by developing solid ionic liquids, but there generally is a trade-off in performance, with the improved mechanical properties balanced by a lowered conductivity.
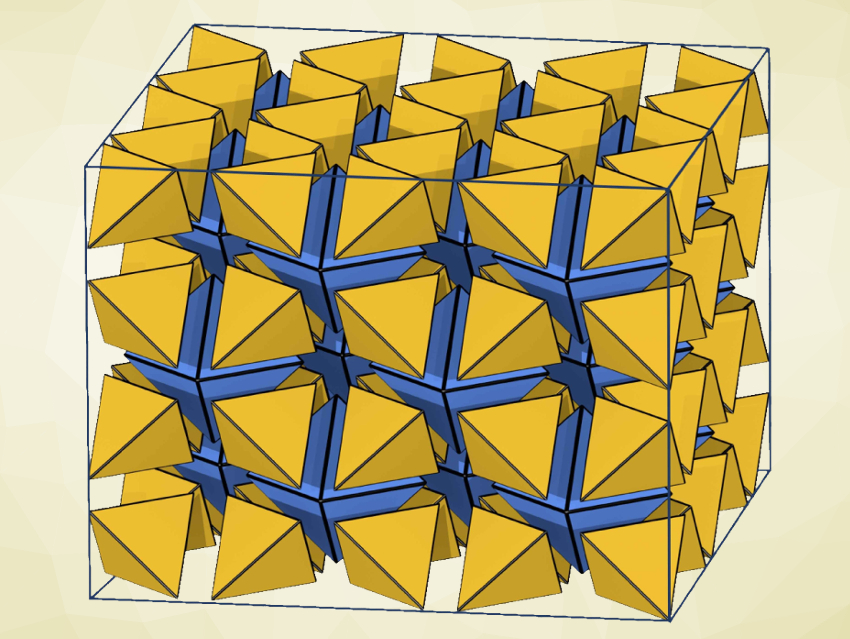
Chong-Qing Wan, Capital Normal University, Beijing, China, Gang Xu, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, and colleagues have tackled these issues by synthesizing a new type of crystalline solid ionic liquid. The solid ionic liquid, named IL1MOF (pictured below on the left), consists of ionic-liquid-type ligands bonded to metal clusters, and thus, features a long-range ordered framework.
The team used a ligand based on 4,4′-biphenyl-dicarboxylate acid (H2BPDC) and 3-(1-methyl-3-imidazolio)propane-3-sulfonate (MIMS) units. The ligand was converted to a MOF by a reaction with ZrCl4 under solvothermal conditions, and CH3SO3H (methanesulfonic acid, MSA) was added to obtain IF1MOF.

The researchers observed an enhanced proton conductivity (two to four orders of magnitude) of the solid ionic liquid compared with its liquid counterpart. The proposed proton transport mechanism is pictured above on the right. No abrupt decrease of conductivity occurred when the temperature was lowered to –40 °C. This work extends applications of ionic liquids to subzero temperatures.
- MOF-Directed Synthesis of Crystalline Ionic Liquid with Enhanced Proton Conduction,
Wen-Long Xue, Wei-Hua Deng, Hui Chen, Rui-Heng Liu, Yukun Li, Lu Wang, Yu-Heng Deng, Wen Hua Li, Ying Yi Wen, Guan-E Wang, Jared M. Taylor, Chong-Qing Wan, Gang Xu,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202010783