Dinitrogen from the air is the basis for artificial fertilizer, and its conversion to ammonia is one of the most widespread industrial processes in the world. However, it consumes an enormous amount of energy and requires the use of transition metals as catalysts and hydrogen gas as a reagent. Holger Braunschweig, University of Würzburg, Germany, and colleagues have developed an N2 fixation reaction that runs at room temperature and uses a main group element as reaction partner with no hydrogen gas involved.
This one-pot reaction represents the first process other than biological fixation that does not involve high temperatures and pressure to fully reduce and protonate the nitrogen.
Everlasting Haber–Bosch Process
The principles of the century-old Haber–Bosch process for converting dinitrogen to ammonia are still used today. Over half of the global population relies on ammonia-based fertilizer made by this process. However, this nitrogen conversion is so energy-intensive that an estimated 2 % of all the world’s energy is consumed in ammonia production. Any alternative process using less energy would allow for considerable savings, both in terms of cost and the use of resources.
In the Haber–Bosch process, a transition metal binds and activates dinitrogen. The activated molecules then react with hydrogen to form hydrogenated nitrogen compounds. The whole process requires high temperatures and pressure. Efforts to make the reduction–protonation reactions less energy-consuming or to use a different catalyst have so far proved fruitless.
Boron and Borylenes
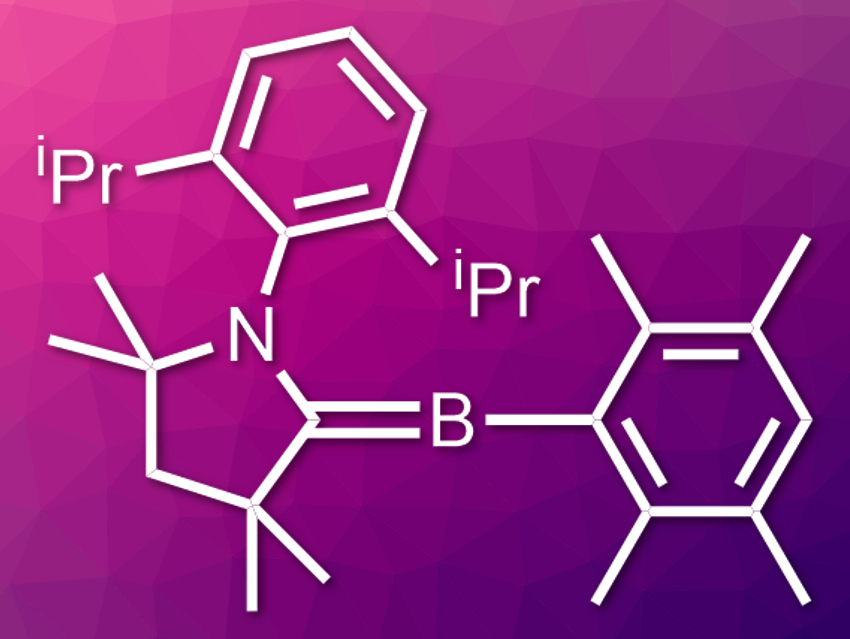
This prompted scientists to look for other elements that could activate the nitrogen. Boron, for example, can form molecules that behave like metals under certain conditions. “We observed that borylenes are able to bind carbon monoxide like a transition metal,” says Holger Braunschweig. Borylenes (example pictured) are low-valency analogs of boranes. To make the borylenes, scientists reduce the boranes and trap the reactive intermediates using electron-deficient substances. These substances can be carbenes, which contain a ligand-stabilized six-electron carbon center.
Borylenes not only bind carbon monoxide; they also react with dinitrogen. The resulting species is a borylene dimer bridged by the dinitrogen. Reduction and protonation produce a hydrazine-bridged radical dimer, which was the starting point for Braunschweig and his team. They thought that further rounds of reduction and protonation would give ammonia as the final product.
Full Reduction
In a first step, the researchers reduced the borylene-bound hydrazine with potassium carbide and protonated the product with boric acid. The isolated species was a borylene amide radical. Further two-electron reduction and protonation yielded a protonated borylene amide. The team also observed that adding trace amounts of water to the reaction mixture produced the borylene amide directly: instead of a two-step reaction, the amide was generated in a one-pot process.
Acid treatment then released an ammonium salt from the borylene. “For the first time, we have made something useful from our fixed nitrogen: ammonia in the form of ammonium chloride,” says Braunschweig. The reaction shows that a full six-electron six-proton reduction of nitrogen is possible without applying high temperature and pressure.
Borylene Reduction Is Not Catalysis
However, the Haber–Bosch process is a catalytic reaction, while the borylene reaction sequence is not. To make the process a sustainable alternative to the metal-catalyzed one, the reaction steps need to be reversible, and the release of ammonia needs to be the driving force. “The strong bond between boron and nitrogen is great if you are only going in one direction, but if you want to have reversibility, it blocks the path,” Braunschweig says. With this in mind, the researchers’ future plans include changing the kinetics of the reaction steps by modifying the borylene ligands.
Alternative nitrogen reductions are still being investigated at the basic research level. Despite the progress made, there is still a long way to go until any nitrogen fixation process other than metal catalysis (or biological fixation) leaves the laboratory and can be scaled up for industry.
- One-pot, room-temperature conversion of dinitrogen to ammonium chloride at a main-group element,
M.-A. Légaré, G. Bélanger-Chabot, M. Rang, R.D. Dewhurst, I. Krummenacher, R. Bertermann, H. Braunschweig,
Nat. Chem. 2020.
https://doi.org/10.1038/s41557-020-0520-6