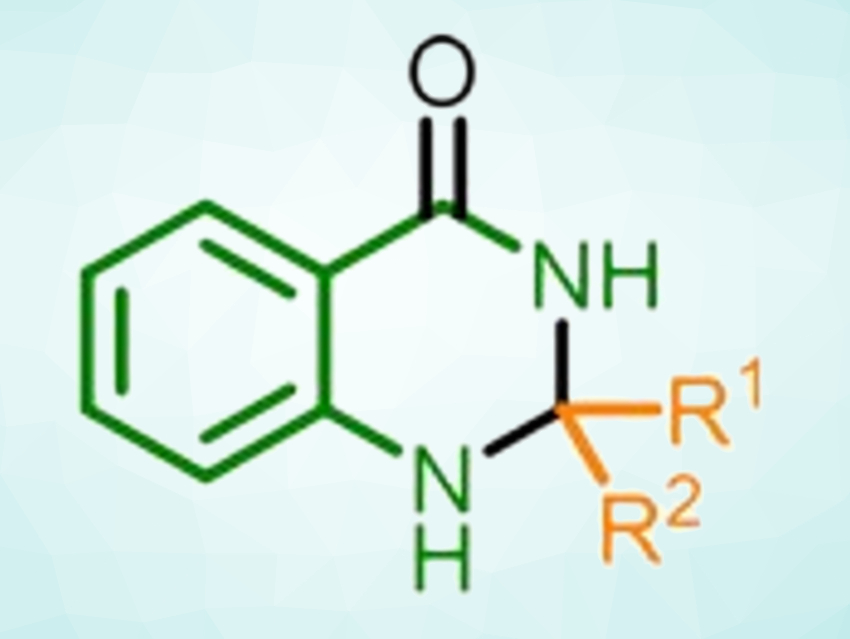
Glucose is an abundant carbohydrate and can be easily obtained from biomass. It can be used as a “green” reductant, e.g., in the synthesis of heterocycles. 2,3-Dihydroquinazolin-4(1H)-ones, for example, are heterocycles with uses in medicinal chemistry. Dihydroquinazolinones can be prepared from various precursors, including 2-aminobenzamides, isatoic anhydride, o-halobenzonitriles, 2-nitrobenzamide, 2-aminobenzonitrile, and 2-nitrobenzonitrile. However, existing processes can require multiple conversion steps or the use of transition-metal catalysts.
Giuliano Cesar Clososki, University of São Paulo, Brazil, Till Opatz, Johannes Gutenberg University, Mainz, Germany, and University of São Paulo, and colleagues have developed a sustainable, one-pot protocol for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones (reaction pictured below). The team used glucose as a green reductant, potassium carbonate as a base, and water as the solvent to react 2-nitrobenzonitrile with a variety of aldehydes and ketones to give the corresponding 2,3‐dihydroquinazolin‐4(1H)‐ones.

The process involves a cascade reaction, starting with the reductive conversion of 2-nitrobenzonitrile to 2-aminobenzamide. An imine formation with the carbonyl compound and a 6-endo-trig-cyclization then give the desired product. The researchers synthesized 18 different 2,3-dihydroquinazolin-4(1H)-ones in yields up to 90 %.
- Glucose as an eco-friendly reductant in a one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones,
Thiago dos Santos, Caroline Grundke, Tobias Lucas, Luca Großmann, Giuliano Cesar Clososki, Till Opatz,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000970