Methanol synthesis is a major industrial process based on the transformation of syngas (a CO/CO2/H2 mixture) over a Cu/ZnO/Al2O3 catalyst. However, despite its widespread use, the reaction mechanism and the nature of the active sites in this process are not fully understood. The reaction could also be useful for decentralized energy storage through methanol synthesis from sustainably derived hydrogen and locally available CO2.
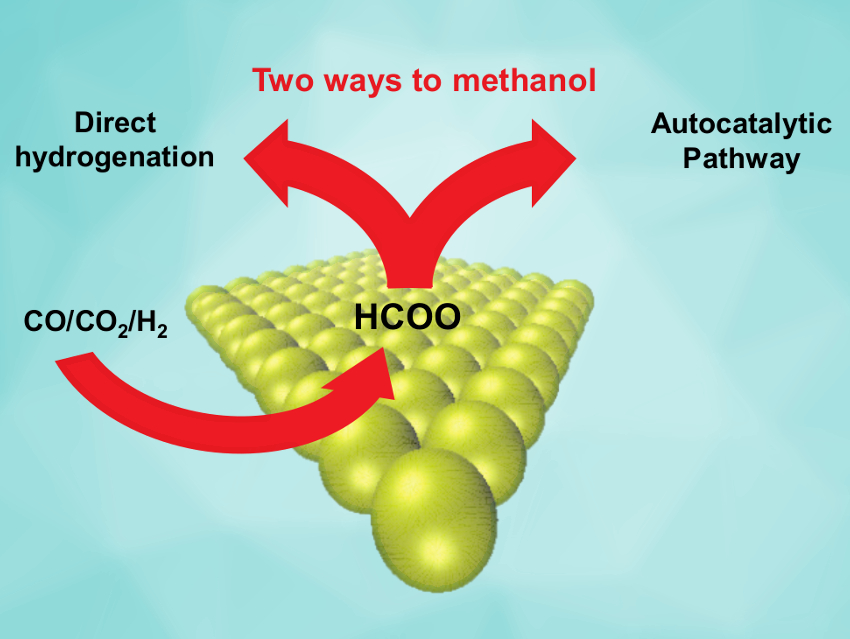
Jakob M. Christensen, Technical University of Denmark, Lyngby, and colleagues have investigated the reaction pathway of methanol synthesis under commercially used conditions with the industrial Cu/ZnO/Al2O3 catalyst. The team monitored the methanol production rates at varying conditions in a flow reactor. They found that the rate increases with rising product concentration. This is a sign of an autocatalytic effect, i.e., the reaction product assists the generation of additional product.
The magnitude of the acceleration means that the autocatalytic pathway is at least several times faster than the direct pathway. This means that the—previously unknown—autocatalytic pathway is responsible for the majority of the methanol produced in the industrial process.
The team proposes a probable autocatalytic pathway that involves the formation of methyl formate ester from methanol and formate on the catalyst surface, followed by hydrogenation of the ester to give methanol. These results could have practical significance because improvements to such a widely used process might have considerable environmental and economic benefits.
- Methanol assisted autocatalysis in catalytic methanol synthesis,
Joachim Thrane, Sebastian Kuld, Niels Dyreborg Nielsen, Anker Degn Jensen, Jens Sehested, Jakob Munkholt Christensen,
Angew. Chem. In. Ed. 2020.
https://doi.org/10.1002/anie.202006921