Supercapacitors have high power densities and long lifespans, but are hampered by their low energy densities. Despite extensive research efforts, the energy densities of supercapacitors are still much lower than those of batteries. The main shortcoming of capacitive charge storage is its low spatial charge density (SCD).
Feng Liu, Chinese Academy of Sciences, Beijing, Liangti Qu, Tsinghua University, Beijing, China, and colleagues have developed an SCD maximization strategy to compensate for this shortage by densely and neatly packing ionic charges in capacitive materials. The team used a highly ordered and compact porous carbon (HOPC) to take up the solvated charge‐carrier ions. HOPC was prepared by hydrothermally reducing graphene oxide (GO) liquid crystals (LCs), followed by densification using capillary forces. The HOPC has an ultrahigh volumetric specific surface area of 1959 m2 cm−3, which allows it to store large amounts of charges.
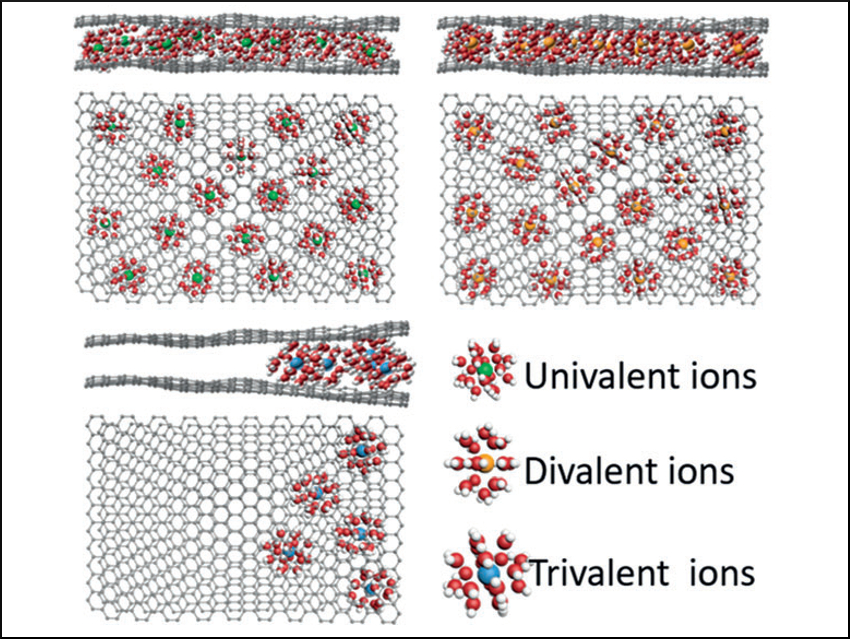
There is a trade-off between the charge of the ions (univalent vs. multivalent) and the size of their hydration shell. The team found that univalent ions (Li+, Na+, K+) can access to the whole active surface area even in the first charge/discharge cycle. Divalent ions (Mg2+, Zn2+) can access to the whole active surface area upon electrochemical activation. However, the trivalent ion Al3+ with its large hydrated radius cannot effectively infiltrate the HOPC even upon repeated activations (pictured).
Using divalent ions as a compromise, a record SCD of ca. 550 C cm–3 was achieved. On this basis, a full-cell hybrid capacitor was constructed that delivers an energy density of 165 Wh L–1 at a power density of 150 W L–1. Even at an ultrahigh power density of 36 kW L–1, it still retains an energy density of 120 Wh L–1. With these performance characteristics, the hybrid capacitor can compete with other state-of-the-art electrochemical energy storage devices.
- Maximization of Spatial Charge Density: An Approach to Ultrahigh Energy Density of Capacitive Charge Storage,
Hongyun Ma, Hongwu Chen, Mingmao Wu, Fengyao Chi, Feng Liu, Jiaxin Bai, Huhu Cheng, Chun Li, Liangti Qu,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202005270