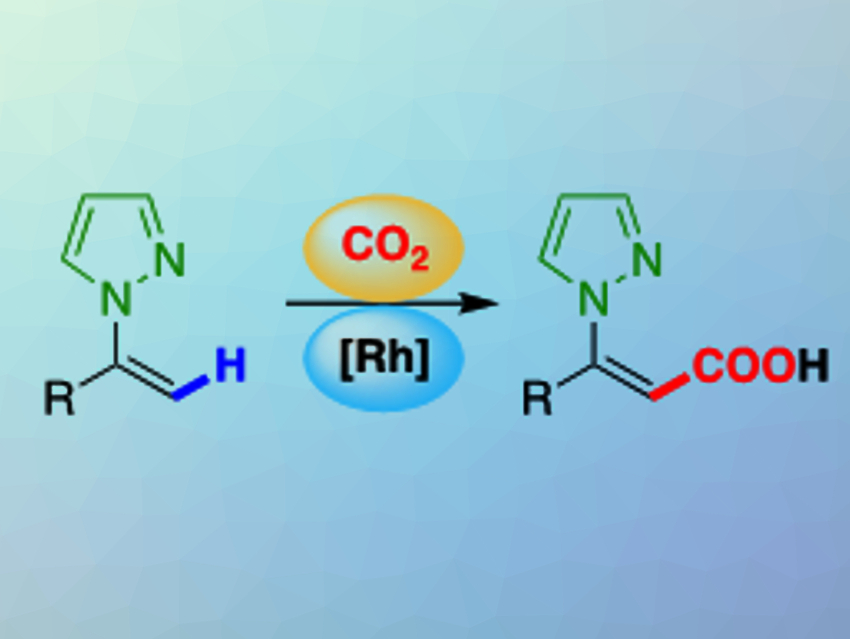
The transition-metal-catalyzed direct carboxylation of C–H bonds with CO2 produces valuable chemicals and is a straightforward method for the utilization of CO2 as a chemical feedstock. Several methods have been developed for the direct carboxylation of aromatic C–H bonds. However, the direct carboxylation of alkenyl C–H bonds has remained largely unexplored, even though this type of reaction could allow the preparation of synthetically useful α,β-unsaturated carboxylic acids.
Nobuharu Iwasawa, Tokyo Institute of Technology, Japan, and colleagues have developed a direct carboxylation of the alkenyl C–H bonds of N-alkenylpyrazoles under a CO2 atmosphere (pictured). The team used a rhodium-based catalyst system consisting of RhCl3·3H2O and trimesitylphosphine (P(Mes)3), together with AlMe2(OMe) as a stoichiometric methylaluminium reagent. Pyrazole was found to be an excellent directing group.
The reaction shows good functional group tolerance and has a wide substrate scope. The pyrazole group in the product can be easily removed or transformed into other functional groups. The carboxylation can be conducted on a gram scale. The researchers are working on extending the reaction to simple alkene substrates.
- Rh-Catalyzed Direct Carboxylation of Alkenyl C–H Bonds of Alkenylpyrazoles,
Takanobu Saitou, Yushu Jin, Kotaro Isobe, Takuya Suga, Jun Takaya, Nobuharu Iwasawa,
Chem. Asian J. 2020.
https://doi.org/10.1002/asia.202000476



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)