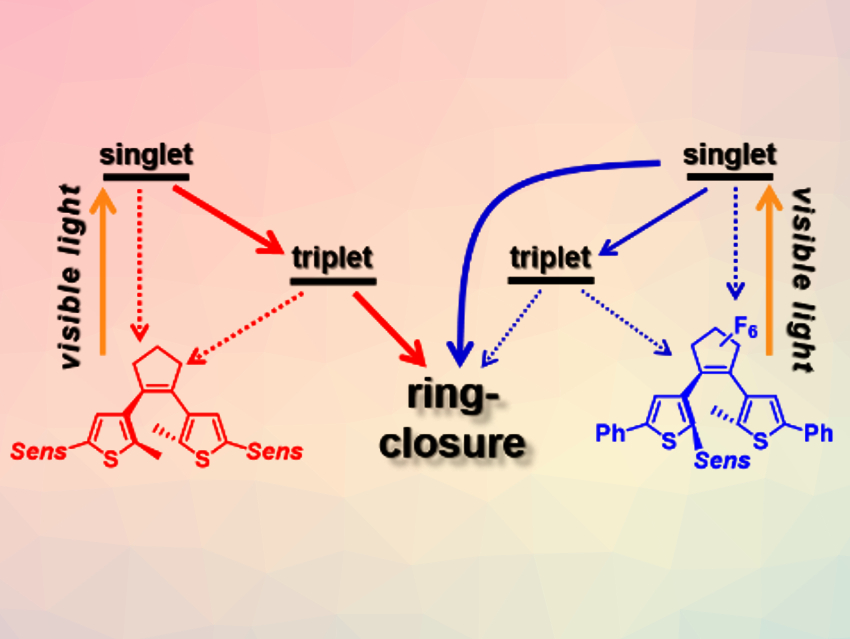
Photoactive materials that can be used in optoelectronic devices need to show a precise and typically repeatable response to light stimuli. Photochromic diarylethenes (DAEs) can be switched between an open and a closed isomer by exposure to light of two different wavelengths. This makes them interesting candidates for, e.g., optical transistors. These applications require a repetitive and reliable operation over many switching cycles. Combining a triplet pathway with visible light excitation can be used to circumvent the reactive singlet state and shift the excitation wavelength to lower energies. This approach (pictured), which is based on using a triplet sensitizer (Sens), can be used to create DAEs with remarkable long‐term photostability.
Stefan Hecht, Humboldt University of Berlin, Germany, DWI Leibniz Institute for Interactive Materials, Aachen, Germany, and RWTH Aachen University, Germany, and colleagues have incorporated heavy-metal-free biacetyl triplet sensitizers into diarylethenes, either at the periphery (pictured in red) or at the photoactive core (pictured in blue). The team investigated these photoswitches using both stationary and transient UV/Vis absorption spectroscopy.
The results indicate that the substitution position is crucial for an efficient switching from the triplet state. The diarylethene with two biacetyl sensitizers at the periphery (pictured in red) is switching predominantly from the triplet excited state, resulting in improved switching stability. In contrast, the diarylethene with one sensitizer at the photoreactive core (pictured in blue) cyclizes from the singlet excited state and shows significantly higher quantum yields for ring-closing and -opening. This work shows that π-conjugation between sensitizer and diarylethene does not necessarily lead to efficient photoswitching from the triplet state. It could help to design functional molecules and materials for optoelectronic devices.
- Mechanistic Insights into the Triplet Sensitized Photochromism of Diarylethenes,
Sebastian Fredrich, Tobias Morack, Michel Sliwa, Stefan Hecht,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202000877