Making complex carbon frameworks in as few steps as possible is one of the overarching goals of organic synthesis. If the reactions can also be conducted using light irradiation, the additional goal of atom economy is achieved. However, light-induced reactions require potent photosensitizers, the best of which are based on expensive rare-metal complexes.
Burkhard König and Alessa B. Rolka, University of Regensburg, Germany suggest replacing expensive iridium-based photosensitizers with purely organic compounds. They have found that some commercially available fluorescent dyes can catalyze dearomatizing rearrangements to a similar extent, and more efficiently, than an established photocatalyst based on the rare metal iridium.
Photosensitizers: Matching Triplet States
Photosensitizers absorb photons of a specific wavelength and convey their energy to a bond in a molecule that needs to be broken. To obtain a unique reaction, the excited energy states in the photosensitizer must be in tune with the energy states of the molecule.
The long-lived, high-energy triplet state of iridium-based systems matches the energy states of the naphthalene and indole aromatic systems. Thus, iridium complexes can be used by synthetic chemists as photosensitizers for interesting photocyclization reactions where the product is a partly dearomatized system. The principle of these reactions is an intramolecular cycloaddition of an alkenyl substituent, e.g., to an activated naphthalene ring. This produces polycyclic frameworks, which are found in many natural products and pharmaceuticals such as steroids and antibiotics.
However, while iridium-based photocatalysts are favored for laboratory purposes, industrial applications require catalysts that are less expensive, less toxic, and soluble in nonpolar solvents, which rules out iridium salts.
OLED-Inspired Organic Photocatalyst
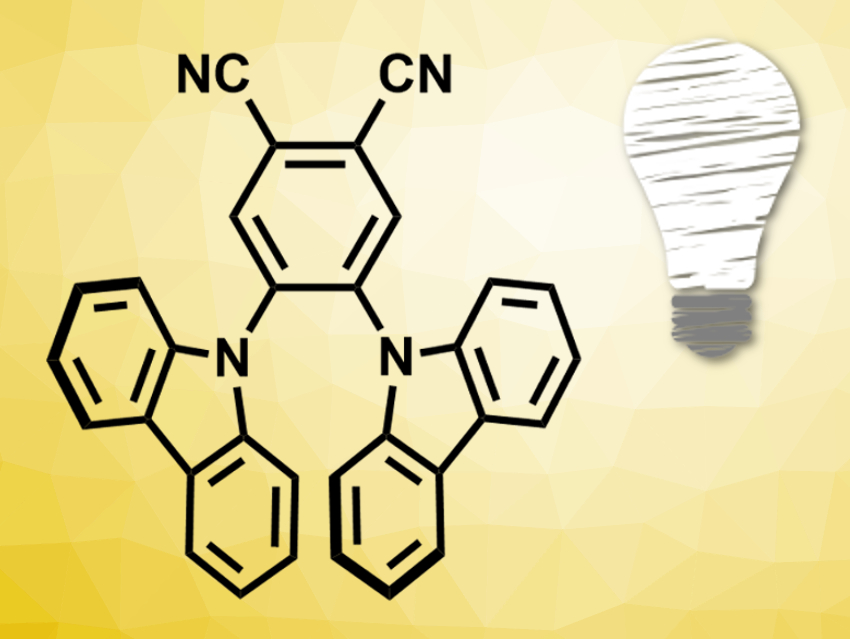
Fluorescent dyes also have a long-lived triplet state and could be used as photosensitizers. Inspired by the huge arsenal of potent fluorescent organic dyes that already exists in the research area of organic light-emitting diodes (OLEDs), the researchers chose the blue-light emitter 4,5-bis(carbazol-9-yl)-1,2-dicyanobenzene (2CzPN, pictured) to set up a standard photocatalyzed cycloaddition reaction with an alkenylated naphthol derivative.
The researchers needed to find a suitable solvent for the reaction. Once they had established chloroform as a convenient solvent, they obtained products in similar or even better yields than with an iridium complex. This was also true when the scientists used alkenylated indoles as substrates: after identifying toluene as a suitable solvent, irradiating the substrates with blue light for a couple of hours produced multiring aminobenzene products of intriguing complexity in high yields.
To generate even more demanding polycyclic products, the team started with allene-substituted indole and naphthol derivatives and obtained dearomatized frameworks containing the highly strained methylenecyclobutane moiety. Compounds containing strained rings and reactive end groups are important starting points for generating even more complex structures.
The researchers highlight that the organic dyes are not only less expensive to produce than rare-metal complexes, but they are also not charged and, therefore, are compatible with nonpolar solvents. By adding substituents to the catalyst, it would be possible to adjust its triplet energy and photocatalytic features. In addition, the 2CzPN dye recyclable and can be purified and reused without affecting performance.
The team advocates for a more dedicated development of organic fluorescent dyes for photocatalytic reactions. They have clearly shown that these dyes, originally developed for OLED applications, can easily match the performance of established rare-metal complexes and are a valid alternative.
- Dearomative Cycloadditions Utilizing an Organic Photosensitizer: An Alternative to Iridium Catalysis,
Alessa B. Rolka, Burkhard König,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c01622