Transforming Electrical Energy into Chemical Energy
With an increase in renewable power production from wind and solar sources and due to their fluctuating nature, it becomes necessary to store excess energy in peak times. Power-to-X concepts are a collection of methods to store electrical energy in the form of chemicals. In these concepts, heterogeneous catalysis plays a key role to allow the effective transformation of electrical energy into chemical energy (e.g., by water electrolysis) or for subsequent chemical reactions with hydrogen (e.g., the methanation of CO2 or the Fischer-Tropsch reaction) [1,2].
Due to the fluctuating nature, a dynamic operation of chemical processes (e.g., load changes) might be necessary or could even be advantageous to increase the catalyst performance, and thus, process efficiency. However, generally it is expected that the dynamic operation of catalysts is more demanding and might enhance catalyst deactivation [2]. The priority program SPP2080 “Catalysts and reactors under dynamic conditions for energy storage and conversion” of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) aims to create a fundamental understanding of catalysts in this regard [3].
One subtopic of this priority program are fundamental studies of catalysts by advanced and operando characterization methods. The latter means that the catalyst is studied during the catalytic chemical reaction under realistic reaction conditions and with simultaneous measurement of the catalytic performance. Such fundamental knowledge about the working catalyst is useful to derive a structure-function relationship of the catalyst, which is the basis for future knowledge-based catalyst design. Grunwaldt et al. and Buurmans et al. have summarized the necessity of advanced characterization techniques for research in heterogeneous catalysis [4,5].
Nickel-Based Catalysts for CO2 Methanation
In my Ph.D. research as part of the SPP2080, I investigate different Ni-based catalysts for the methanation of CO2 (CO2 + 4 H2 ⇄ CH4 + H2O) by different time- and spatially resolved methods. A combination of different techniques is essential, because the length- and time-scales relevant for transport and deactivation effects in heterogeneous catalysis cover multiple orders of magnitudes, as illustrated in Fig. 1 [2]. The main focus of my studies concerns 3D spatially-resolved investigations of the catalysts by electron and X-ray tomography techniques, complemented with operando X-ray absorption spectroscopy (XAS) and operando X-ray tomography experiments.
In a simplified picture, one can imagine a typical catalyst consisting of a support material, which looks like a sponge, and small nanoparticles deposited on this support, which form the active sites. The pore system of such materials is important for the mass transport of reactants to the active sites. The performance of the catalyst is not only limited by the activity of the active site, but also by the mass transport of products and reactants. For this reason, a detailed understanding of the pore system of catalysts is required. They are typically characterized using N2-sorption or Hg-porosimetry methods, often assuming a cylindrical pore shape only resulting in 1D information. Both methods only allow a simplified model for the pores of the catalysts. However, if you think about a sponge you would not expect a homogeneous pore system.
 |
|
Figure 1. Relevant length- and time-scales for transport and deactivation effects in heterogeneous catalysis and selected methods with respective covered ranges. |
Imaging Pore Networks
In my Ph.D. research, I use electron tomography (ET) and ptychographic X-ray computed tomography (PXCT) to study the pore structure on different length scales. The combination of these two methods is essential to address different pore sizes. According to the IUPAC definition, one distinguishes three different pore types: micro- (<2 nm), meso- (2–50 nm) and macropores (>50 nm). ET allows a resolution down to 1 nm, and could theoretically resolve all three length scales. However, the sample size is limited to a few hundred nanometers, which does not allow the statistically relevant investigation of macropores or large mesopores. Here, PXCT comes into play. This method allows the investigation of samples up to some tens of micrometers in size at resolutions down to 10–20 nm.
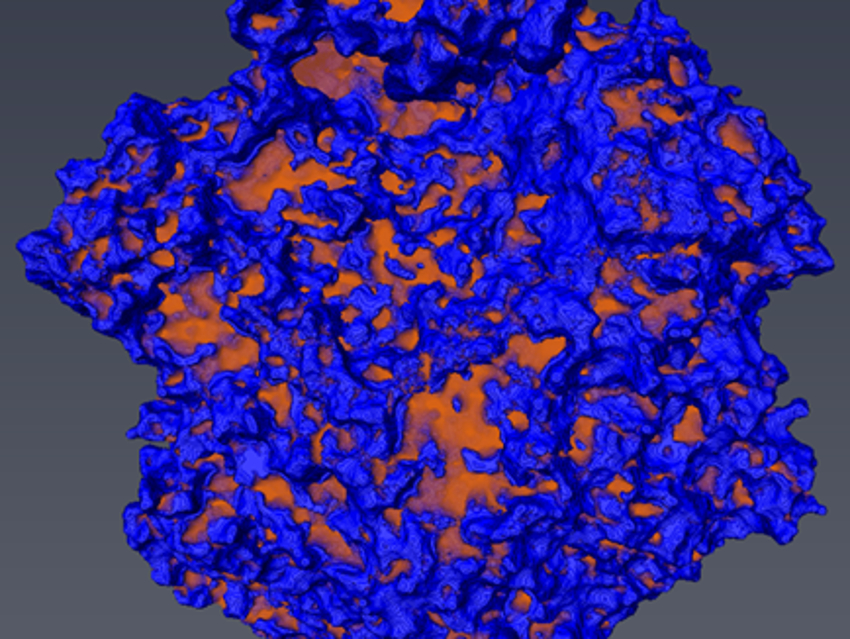
With a combination of the two methods, we can investigate all relevant length scales and obtain a 3D visualization of the pore network. In Fig. 2, an image of an ET of a single Ni/Al2O3 catalysts particle is shown as an example. By image analysis of the tomograms, one can derive a pore-network model and descriptors for mass-transport properties.
 |
|
Figure 2. Rendered ET image of an Ni/Al2O3 catalyst; material in blue and pores in orange. |
Challenges and Outlook
The most challenging part of the studies are the data analysis process and the limited access to experimental facilities. For ET experiments, regular access to a suitable transmission electron microscope is required, while PXCT experiments need to be performed at dedicated beamlines at a synchrotron radiation source. The data analysis and experiments require strong interdisciplinary work with physicists, while chemical engineers can use the measures of the pore system for modelling and simulation. This interdisciplinary work is challenging and exciting at the same time and one of the points I most enjoy in my work.
In the future, experiments are planned which not only investigate the 3D pore structure of the catalysts, but also the chemical properties of the materials. This involves X-ray tomography studies with different contrast modes, such as X-ray diffraction to study present crystalline phases and their distribution or X-ray absorption spectroscopy to investigate the chemical state, e.g., the oxidation state of the active site. A combination of the results of all studies leads to a holistic understanding of the Ni-based methanation catalysts, showing the power of time- and spatially resolved methods for catalysis research.
References
- [1] The Revolution Continues: Energiewende 2.0,
R. Schlögl,
Angew. Chem. Int. Ed. 2015, 54, 4436–4439.
https://doi.org/10.1002/anie.201405876 - [2] Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions,
K. F. Kalz, R. Kraehnert, M. Dvoyashkin, R. Dittmeyer, R. Gläser, U. Krewer, K. Reuter, J.-D. Grunwaldt,
ChemCatChem 2017, 9, 17–29.
https://doi.org/10.1002/cctc.201600996 - [3] SPP 2080 DynaKat: Project Areas,
www.itcp.kit.edu. (accessed June 18, 2020) - [4] Imaging Catalysts at Work: A Hierarchical Approach from the Macro- to the Meso- and Nano-scale,
J.-D. Grunwaldt, J. B. Wagner, R. E. Dunin-Borkowski,
ChemCatChem 2013, 5, 62–80.
https://doi.org/10.1002/cctc.201200356 - [5] Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy,
I. L. C. Buurmans, B. M. Weckhuysen,
Nat. Chem. 2012, 4, 873–886.
https://doi.org/10.1038/nchem.1478
Author
Sebastian Weber
Institute of Catalysis Research and Technology, Karlsruhe Institute of Technology (KIT), Eggenstein-Leopoldshafen, Germany