Methanol Synthesis over Copper Catalysts
The production of methanol from CO and H2 is a widely used process in industry since the 1920s. The use of CO2 is also feasible, either by a prior reverse water-gas shift reaction (CO2 + H2 ⇌ CO + H2O) or via the direct hydrogenation of CO2. This offers a sustainable production pathway to methanol—an important base chemical and fuel—by the valorization of waste CO2. It could also allow the use of methanol as a storage molecule for hydrogen produced from renewable energy sources via water electrolysis [1]. However, in this scenario, the process and especially the catalyst would have to deal with a fluctuating gas supply, constantly changing the redox potential of the synthesis gas mixture.
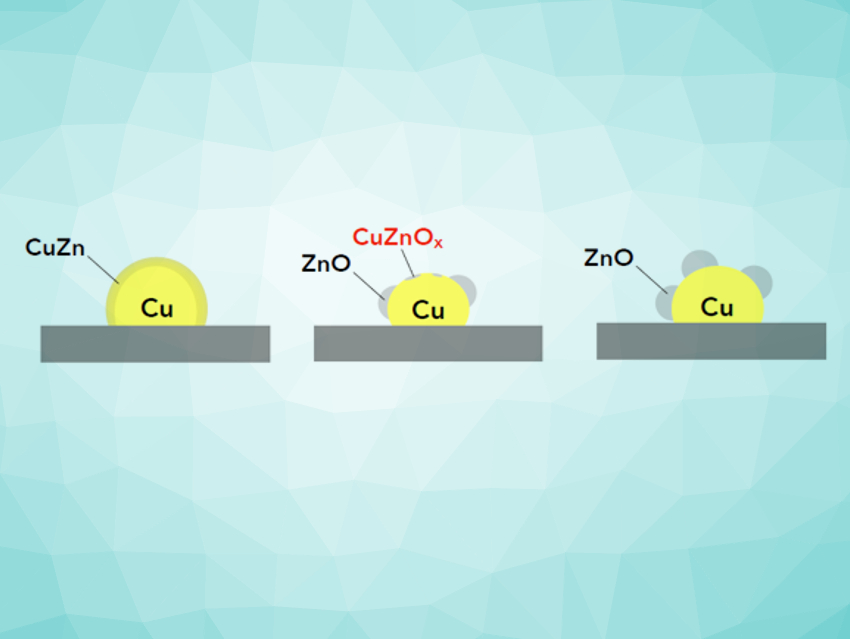
Since the implementation of the low-pressure methanol process by Imperial Chemical Industries (ICI), UK, in the 1960s, the typical industrial catalyst for methanol synthesis is a Cu/ZnO catalyst with additional low-content promotion by Al or Mg. In this material, ZnO serves both as the support and an electronic promoter. This promotional effect is derived from a dynamic accumulation of ZnOx species (0 ≤ x < 1) on the copper particle surface and is described as strong metal–support interactions (SMSI) [2]. Al2O3 is seen as a structural promoter for the ZnO support, but an additional effect of electronic promotion is discussed as well [3].
 |
|
Figure 1. Schematic drawing of the dynamics in the Cu/ZnO system as a function of the chemical potential of the feed gas (depending on the CO:CO2 ratio in the COx/H2 synthesis gas). The actual eligible configurations of ZnO, i.e., oxygen-deficient ZnOx (x<1) or surface alloy islands on Cu, can be monitored and discriminated spectroscopically, whereas the fully oxidized or reduced states are theoretical reference points. |
The actual nature of the ZnOx species and the catalytically active phases are subject to discussion. They are highly dependent on the redox potential of the gas feed and can influence the reaction to methanol at the catalyst surface (see Fig. 1).
Having said that, it becomes clear that it is very important to study the catalyst’s structure and performance under dynamic conditions. In the framework of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) priority program SPP2080, we adress these dynamic interactions using synthetic, in situ– and operando-analytical as well as computational methods for different promoter oxides [4–6].
Using MgO Instead of ZnO
The synthesis and catalytic performance of Cu/ZnO/Al2O3 catalysts is well documented in the literature [7,8]. However, to study the promotional effect of ZnO, we need to separate this from the support function that ZnO also has in the classical system. Therefore, we developed a model system with the irreducible oxide MgO instead of ZnO.
During the synthesis of these materials, the co-precipitation yields mixed Cu,Mg hydroxycarbonates of the malachite or mcguinnessite structure type. Calcination and subsequent reduction lead to Cu/MgO catalysts. These materials are active in CO hydrogenation, but it is possible to add ZnO in small amounts by impregnation, re-enabling the hydrogenation of CO2 and CO/CO2 mixtures [9]. This offers the opportunity to investigate the state and dynamic changes of the ZnO promoter with element-specific X-ray absorption spectroscopy under dynamic reaction conditions, which we currently do together with our collaborators from the Karlsruhe Institute of Technology (KIT).
Precursor Chemistry of Cu/MgO Catalysts
Apart from the spectroscopic and computational investigations of the ZnO promotion, we are also interested in the precursor chemistry of these Cu/MgO catalysts from a solid-state chemical point of view: Crystallographically, the mineral mcguinnessite, (Cu0.5Mg0.5)2CO3(OH)2, belongs to the malachite structure group. However, as its Cu,Zn equivalent rosasite, it is not structurally identical to the pure Cu malachite, Cu2CO3(OH)2, but shows a slightly different crystal structure without a smooth transition from one to the other [10].
This leads to interesting findings when we gradually substitute Cu by Mg in a series of synthesis experiments: Typically, the hydroxycarbonates are synthesized by pH-controlled co-precipitation at 65 °C in an automated lab reactor. The precipitates are aged in the mother liquor, filtered, washed, and dried. When using small amounts of Mg (up to 10 at-%), we obtain moderately crystalline (partly Mg-substituted) malachites of the typical green color. In these experiments, a characteristic pH minimum appears in the synthesis protocol after a given time and is used as an indicator for successful crystallization—this aging time scales nearly linearly with the amount of Mg used. However, for larger ratios (e.g., 15 or 30 at-% Mg), another rather step-like pH drop is observed much earlier and only amorphous products of blue color could be isolated. These products are prone to Mg leaching during the washing step.
In a different approach, we applied a hydrothermal aging step at pH 8.0 (102 °C, 20 h) and were able to obtain turquoise-colored crystalline mcguinnessites—where Cu is substituting Mg in deviation from the regular 1:1 ratio (see Fig. 2). Hence, we expect a changeover between the formation of Mg-substituted malachite and Cu-substituted mcguinnessite at a point between 10 and 15 at-% Mg.
 |
|
Figure 2. Left: Schematic synthesis protocol with different shapes of pH drops during aging. Center: Ageing times increase with rising Mg content; for high amounts, amorphous products are formed after a few hours; so far, longer aging times led to the oxolation of Cu to CuO which is to be avoided during the wet-chemical synthesis. Right: PXRD patterns of the synthesized Cu,Mg hydroxycarbonates and comparison of the crystal structures. |
Further experiments in the respective composition range as well as a thorough investigation of the hydrothermal treatment will help elucidate the crystal chemistry of these mixed Cu,Mg hydroxycarbonates. This will improve our knowledge about the possibilities to substitute Cu and Mg in the malachite and mcguinnessite crystal structures, and should allow us to optimize the synthesis and preparation of Cu/MgO catalysts from crystalline precursors.
Acknowledgment
We gratefully acknowledge the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) for funding through the priority program SPP2080 “Catalysts and reactors under dynamic conditions for energy storage and conversion” (project number 406903668) and especially Jan-Dierk Grunwaldt and Felix Studt at KIT for fruitful discussions and an interactive and lively collaboration.
References
- [1] Beyond Oil and Gas: The Methanol Economy,
G. A. Olah,
Angew. Chem. Int. Ed. 2005, 44, 2636–2639.
https://doi.org/10.1002/anie.200462121 - [2] Formation of a ZnO Overlayer in Industrial Cu/ZnO/Al2O3 Catalysts Induced by Strong Metal-Support Interactions,
T. Lunkenbein, J. Schumann, M. Behrens, R. Schlögl, M. G. Willinger,
Angew. Chem. Int. Ed. 2015, 54, 4544–4548.
https://doi.org/10.1002/anie.201411581 - [3] Performance Improvement of Nanocatalysts by Promoter-Induced Defects in the Support Material: Methanol Synthesis over Cu/ZnO:Al,
M. Behrens, S. Zander, P. Kurr, N. Jacobsen, J. Senker, G. Koch, T. Ressler, R. W. Fischer, R. Schlögl,
J. Am. Chem. Soc. 2013, 135, 6061–6068.
https://doi.org/10.1021/ja310456f - [4] Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions,
K. F. Kalz, R. Kraehnert, M. Dvoyashkin, R. Dittmeyer, R. Gläser, U. Krewer, K. Reuter, J.-D. Grunwaldt,
ChemCatChem 2017, 9, 17–29.
https://doi.org/10.1002/cctc.201600996 - [5] In Situ Investigations of Structural Changes in Cu/ZnO Catalysts,
J.-D. Grunwaldt, A. M. Molenbroek, N.-Y. Topsøe, H. Topsøe, B. S. Clausen,
J. Catal. 2000, 194, 452–460.
https://doi.org/10.1006/jcat.2000.2930 - [6] The Mechanism of CO and CO2 Hydrogenation to Methanol over Cu-Based Catalysts,
F. Studt, M. Behrens, E. L. Kunkes, N. Thomas, S. Zander, A. Tarasov, J. Schumann, E. Frei, J. B. Varley, F. Abild-Pedersen, J. K. Nørskov, R. Schlögl,
ChemCatChem 2015, 7, 1105–1111.
https://doi.org/10.1002/cctc.201500123 - [7] How to Prepare a Good Cu/ZnO Catalyst or the Role of Solid State Chemistry for the Synthesis of Nanostructured Catalysts,
M. Behrens, R. Schlögl,
Z. Anorg. Allg. Chem. 2013, 639, 2683–2695.
https://doi.org/10.1002/zaac.201300356 - [8] Synthesis and Characterisation of a Highly Active Cu/ZnO:Al Catalyst,
J. Schumann, T. Lunkenbein, A. Tarasov, N. Thomas, R. Schlögl, M. Behrens,
ChemCatChem 2014, 6, 2889–2897.
https://doi.org/10.1002/cctc.201402278 - [9] The Role of the Oxide Component in the Development of Copper Composite Catalysts for Methanol Synthesis,
S. Zander, E. L. Kunkes, M. E. Schuster, J. Schumann, G. Weinberg, D. Teschner, N. Jacobsen, R. Schlögl, M. Behrens,
Angew. Chem. Int. Ed. 2013, 52, 6536–6540.
https://doi.org/10.1002/anie.201301419 - [10] Crystal structure determination and Rietveld refinement of rosasite and mcguinnessite,
N. Perchiazzi,
Z. Kristallogr. 2006, 2, 505–510.
https://doi.org/10.1524/zksu.2006.suppl_23.505
Authors
Gereon Behrendt*![]() , Malte Behrens
, Malte Behrens![]()
University of Duisburg-Essen, Inorganic Chemistry, Essen, Germany
*Corresponding author: [email protected]