Power-to-X Concepts
Achieving the energy transition requires the shift from fossil and nuclear fuels towards renewable energy sources, such as solar and wind power. However, due to the fluctuating availability of renewable energy sources, there will be periods of energy surplus and periods of energy shortage. Efficient methods for energy storage are necessary to compensate for these fluctuations. In this context, power-to-X concepts are currently under intensive investigation.
Power-to-X concepts aim at the storage of electrical energy in chemical compounds. An example is hydrogen obtained by water electrolysis. Due to the lack of hydrogen infrastructure, it is useful to convert hydrogen into methane (synthetic natural gas) by adding carbon dioxide and using catalytic fixed-bed reactors. The produced methane can then be injected into the existing natural gas network. The consumption of carbon dioxide also reduces greenhouse gas emissions.
However, the optimal operation of catalytic fixed-bed reactors for carbon dioxide methanation is a technological challenge. Under certain circumstances, the exothermicity and temperature-dependence of the methanation reaction can lead to uncontrollable reactor conditions, known as thermal runaway. In this case, the heat release of the chemical reaction increases the reactor temperature, which further accelerates the reaction rate, and thus, also the heat-release rate.
This self-reinforcing effect can be so pronounced that even the intensive reactor cooling of multi-tubular fixed-bed reactors is insufficient. The result is a distinct temperature profile along the axis of the reactor. The peak of the temperature profile is known as a hotspot. Hotspots can severely exceed the temperature limits of the reactor material and catalyst. Consequently, the avoidance of runaway conditions has the highest priority for the safe operation of fixed-bed reactors. In addition, there are requirements with regard to product purity, so that the synthetic natural gas can be injected into the natural gas grid without excessive post-treatment [1].
Core-Shell Catalyst Particles
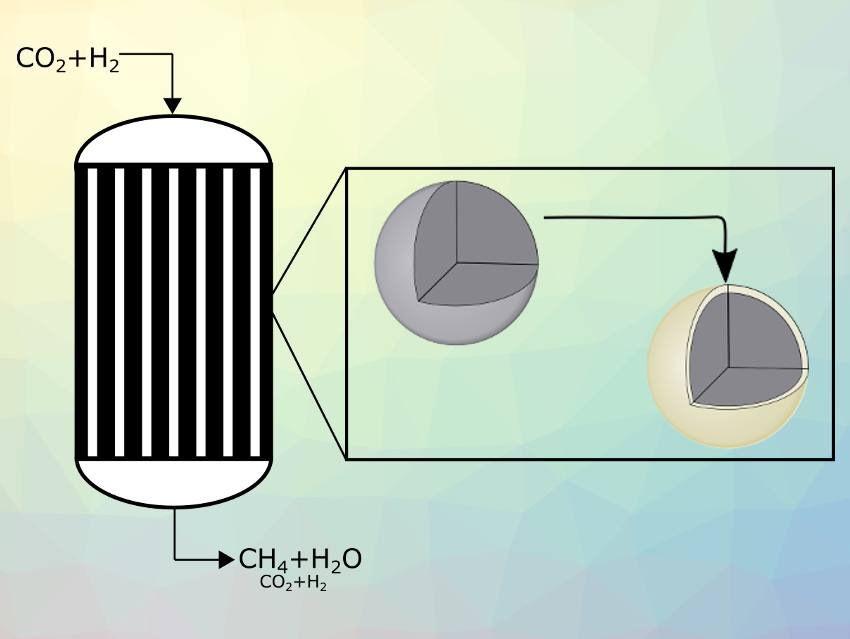
The heat generation in the reactor is inseparably linked to the effective reaction rate of the catalyst particles. This rate is influenced by the active material within the particles, but also by the particle structure. In this context, a catalyst particle design consisting of a catalytically active core surrounded by an inert shell has proven to be advantageous in comparison with state-of-the-art uniform catalyst particles [2]. At low temperatures only, the active core is rate-determining, and the additional shell has no significant influence. Thus, both concepts show almost the same effective reaction rate.
This changes at higher temperatures, where the inert shell acts as a mass-transport barrier and becomes rate-determining. Due to different temperature-dependences for diffusion and reaction rate, the inert shell prevents the catalyst particle from exceeding a certain effective reaction rate (shown in the Arrhenius plot in Fig. 1). Therefore, the self-reinforcing effect that can lead to reactor runaway is prevented, as reactor cooling remains sufficient even at high temperatures. Towards the colder reactor outlet, high reaction rates can be maintained, and thus, allow for high methane yields.
 |
|
Figure 1. Arrhenius plots of catalyst particles with and without inert shell (left) and state space trajectories of a fixed-bed reactor filled with these particles (right). The results shown are based on Matlab simulations described in [2]. The upper catalyst particle (pictured in silver) is state-of-the-art and can lead to the described runaway behavior. The lower catalyst particle (shell pictured in gold) is the core-shell particle which can be used to avoid this. |
The use of the novel catalyst particle design is also advantageous for flexible reactor operation, e.g., due to volatile electricity, and thus, reactant supply. Even large fluctuations in inlet velocity and reactor pressure only lead to slight changes in hotspot temperature and product quality. A detailed analysis is given in [2].
Since the previous results are based on simulation studies, the realization of the discussed catalyst particle concept is of great interest. In the long term, the validation of the results on the laboratory and pilot-plant scales is planned.
Acknowledgments
This research work was conducted within the DFG Priority Program SPP2080 “Catalysts and reactors under dynamic conditions for energy storage and conversion” (funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 406914011).
Ronny Zimmermann and Jens Bremer are also affiliated with the International Max Planck Research School (IMPRS) for Advanced Methods in Process and Systems Engineering, Magdeburg, Germany (funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 406914011).
References
- [1] Operation range extension via hot-spot control for catalytic CO2 methanation reactors,
Jens Bremer, Kai Sundmacher,
React. Chem. Eng. 2019, 4, 1019–1037.
https://doi.org/10.1039/c9re00147f - [2] Optimal catalyst particle design for flexible fixed-bed CO2 methanation reactors,
Ronny Tobias Zimmermann, Jens Bremer, Kai Sundmacher,
Chem. Eng. J. 2020, 387, 123704.
https://doi.org/10.1016/j.cej.2019.123704
Authors
Ronny Tobias Zimmermann1, Jens Bremer2, Kai Sundmacher1,2*
1 Otto von Guericke University Magdeburg, Chair for Process Systems Engineering, Universitätsplatz 2, 39106 Magdeburg, Germany
2 Max Planck Institute for Dynamics of Complex Technical Systems, Dpt. Process Systems Engineering, Sandtorstraße 1, 39106 Magdeburg, Germany
*Corresponding author: [email protected]




The inert shell catalyst will promise the operating temperature up to 773deg.K for 8000hrs continuous operation?