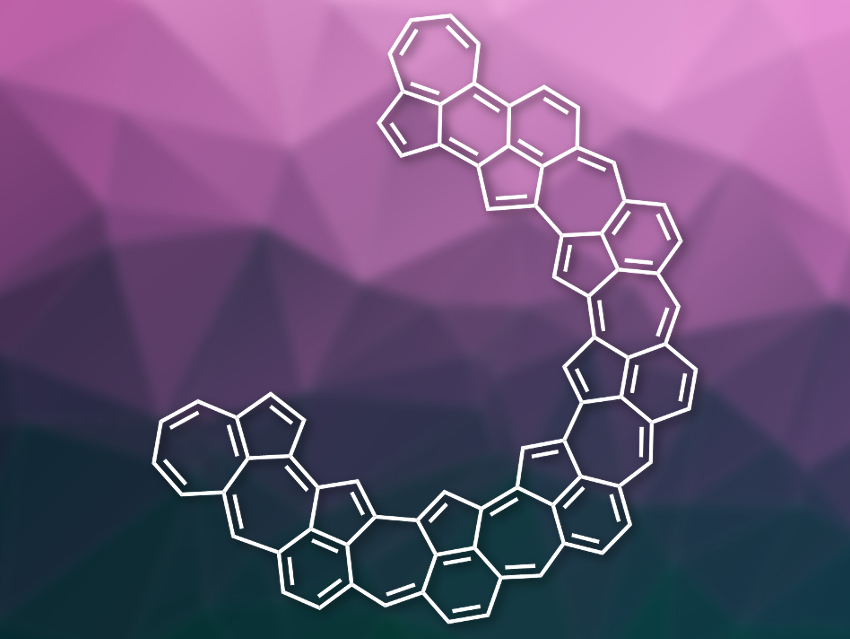
A pure polyazulene network has so far remained hidden from scientists. However, Akimitsu Narita, Max Planck Institute for Polymer Research, Mainz, Germany, and Okinawa Institute of Science and Technology Graduate University, Kunigami, Japan, Roman Fasel, Swiss Federal Laboratories for Materials Science and Technology (Empa), Dübendorf, and University of Bern, Switzerland, Klaus Müllen, Max Planck Institute for Polymer Research and University of Mainz, Germany, and colleagues have come close: By fusing pentagon–heptagon pairs on a gold surface, the team was able to synthesize a fully condensed, unsaturated polygonal carbon system.
With only one fused hexagon per azulene unit remaining, it is the largest condensed non-hexagonal carbon network to date. Despite its unsaturated character, the electronic structure of this network is largely nonaromatic.
Azulenes
 Azulene (pictured on the right) is an aromatic compound with a deep blue color that is naturally occurring. The molecule is a naphthalene isomer consisting of two condensed carbon rings: one five-membered, the other seven-membered, rather than the usual two six-membered rings of naphthalene. Although its electron system is unsaturated and aromatic, azulene is less stable than naphthalene.
Azulene (pictured on the right) is an aromatic compound with a deep blue color that is naturally occurring. The molecule is a naphthalene isomer consisting of two condensed carbon rings: one five-membered, the other seven-membered, rather than the usual two six-membered rings of naphthalene. Although its electron system is unsaturated and aromatic, azulene is less stable than naphthalene.
In theory, a polyazulene network would contain more fused pentagonal and heptagonal rings instead of multiple hexagons as in graphene and graphite. However, such a structure is expected to be highly unstable and has not yet been observed.
From Azulenes to Fused Azulenes
Non-hexagonal fused rings are a common defect found in graphene, where they disrupt its continuous electronic structure and form grain boundaries within the hexagonal lattice. Understanding the structural and electronic properties of unsaturated fused carbon rings other than hexagons could be of use in constructing flawless graphene or in designing new organic electronic materials.
The researchers investigated synthetic routes to azulene networks. They started the fused azulene synthesis by annealing biazulenyls on a gold surface. This produced long chains of pentyl-ring-connected polyazulenes, which could be observed by scanning electron microscopy. The gold atoms appeared to be involved in connecting the pentyl rings of the azulene monomers.
Further annealing on the gold surface turned these polymers into more compact curved strings (example pictured), which under the scanning transmission microscope look a bit like curled-up worms pressed onto a surface. The half-ring structures, with radii of roughly a tenth of a nanometer, fully consist of fused carbon polygons, as revealed by atomic force microscopy. About one third of the polygons were pentagons, another third heptagons, with the remainder made up of hexagons. “Notably, all pentagons appeared in the inner part of the curved structure, with the hexagons staying in the outer and the heptagons in the middle parts,” the team observed.
This means that the azulene molecules were fused to give an array of condensed pentagon–hexagon pairs, with the pentagons pointing inwards on the curved structure and the hexagons attached at the outside, filling the gaps between the heptagons. To explain the transformation of the straight polymeric chains to the curled-up worm structure, the researchers suggest a sequence of radical rearrangement steps both within and between the azulene molecules, catalyzed by the gold surface.
Unsaturated and Flat, But Not Aromatic
As the driving force behind this rearrangement, the researchers identified the creation of a fully interconnected sp2-carbon skeleton. However, despite their all-sp2 character, the multiple-fused polygons showed rather low aromaticity, as was shown by calculations of the NMR shifts and ring currents. The team was surprised that only the pentagons could be identified as aromatic, with the rest of the polygons not showing significant ring currents. Furthermore, the molecules are polar, with regions of higher and lower electron density.
With the first synthesis of a fully condensed polyazulene, the researchers have introduced a new carbon-based morphology to the family of graphene, nanorods, and fullerenes. As a next step, they want to find out more about its electronic structure to explore its usefulness in organic electronics applications.
- On-Surface Synthesis of Unsaturated Carbon Nanostructures with Regularly Fused Pentagon-Heptagon Pairs,
I. C.-Y. Hou, Q. Sun, K. Eimre, M. Di Giovannantonio, J. I. Urgel, P. Ruffieux, A. Narita, R. Fasel, K. Müllen,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c03635