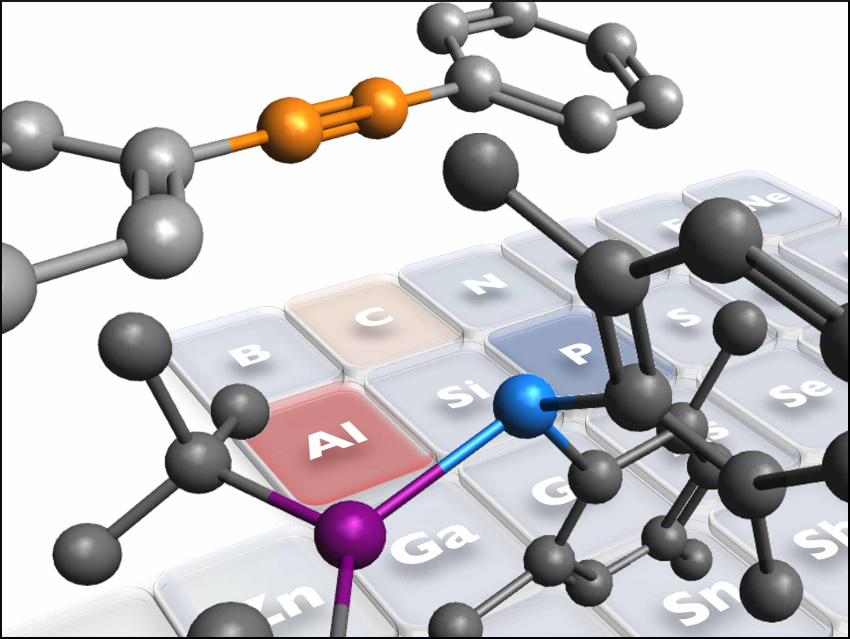
Phosphanylalumanes, i.e., P–Al single-bonded species, can combine the functions of isolated Lewis acids (LA) and Lewis bases (LB). This is similar to the principle of frustrated Lewis pairs (FLPs), in which a compound or mixture contains a Lewis acid and a Lewis base that cannot combine to form an adduct due to steric hindrance. Many FLPs can activate small molecules such as CO2 or H2.
Yoshiyuki Mizuhata, Kyoto University, Japan, and colleagues have found that phosphanylalumanes can be used for the activation of alkynes under mild conditions. The team reacted a λ3,λ3‐phosphanylalumane (pictured below on the left) with alkynes in benzene. The resulting adducts (pictured below on the right) are intramolecular FLPs.

These alkyne adducts can undergo typical FLP reactions, such as reversible binding with CO2. The team found that the adducts can also react with dimethyl acetylenedicarboxylate (DMAD) to give unique fused ring compounds (pictured below). According to the researchers, they will study further small-molecule activation reactions using phosphanylalumanes.

- Additive‐Free Conversion of Internal Alkynes by Phosphanylalumanes: Production of Phosphorus/Aluminum Frustrated Lewis Pairs,
Tatsuya Yanagisawa, Yoshiyuki Mizuhata, Norihiro Tokitoh,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000239