Halogen bonds (XBs) are noncovalent interactions between a halogen substituent with an electrophilic region and a nucleophilic Lewis base. The origin of these interactions has been discussed controversially. Halogen bonds are sometimes thought to be purely electrostatically driven. This makes adducts between an anionic halogen-bond donor and another anion seem counterintuitive. However, orbital interactions could also contribute significantly and might allow the formation of “anti‐electrostatic” XBs (AEXBs). AEXBs had been predicted theoretically but, so far, there had been no experimentally verified examples between an organic XB donor and another anion.
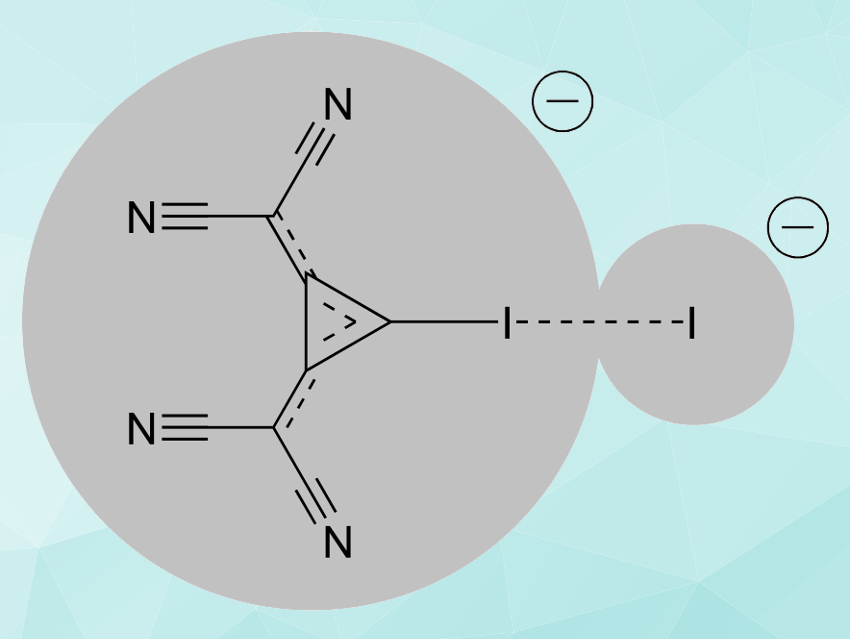
Stefan M. Huber, University of Bochum, Germany, Robert Weiss, University of Erlangen-Nuremberg, Erlangen, Germany, and colleagues have synthesized compounds which show that XBs can also exist between two anions. The team synthesized a iodinated 1,2-bis(dicyanomethylene)cyclopropanid derivative (pictured above on the left) from its triethylammonium analogue via a reaction with sodium borohydride and a iodination with 1,3‐diiodo‐5,5‐dimethylhydantoin. A cation exchange from sodium to the organic cation tris(dimethylamino)cyclopropenium (TDA) was used to increase the solubility of the salt in organic solvents.
The researchers prepared three halogenated salts based on the 1,2-bis(dicyanomethylene)cyclopropanid scaffold by adding TDAX (X = I, Br, Cl). The products (example pictured) were characterized using X-ray diffraction. The crystal structures show AEXBs between two anions. This work highlights the importance of polarization and orbital interactions for halogen bonding. It could be a step towards the use of new classes of anionic XB donors.
- “Anti-Electrostatic” Halogen Bonding,
Jana Holthoff, Elric Engelage, Robert Weiss, Stefan Matthias Huber,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202003083