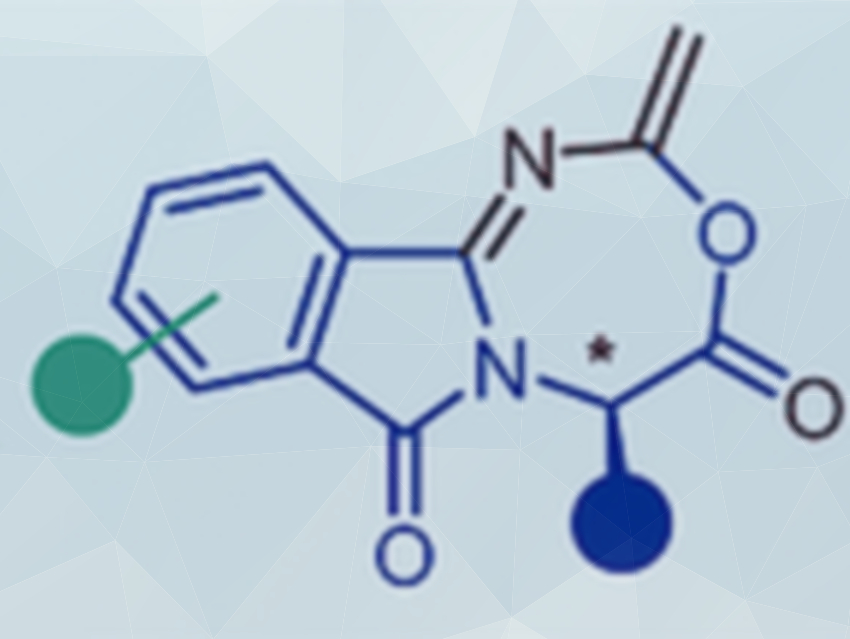
The preparation of heterocyclic compounds is important in synthetic chemistry, e.g., for drug design and materials science. Nuno Maulide and colleagues, University of Vienna, Austria, have discovered a synthesis approach for a new class of fused seven-membered heterocycles (pictured above).
The reaction relies on α‐phthalimido‐amides, which are readily prepared from amino acids in two simple reactions steps. The respective amino acid is first reacted with phthalic anhydride to form the phthalimido group and then with thionyl chloride and HNMe2 to give the desired α‐phthalimido‐amide (pictured below on the left). When these intermediates are reacted with trifluoromethanesulfonic anhydride using 2,6‐di-tert‐butylpyridine (DTBP) as a base and acetonitrile as a solvent, the solvent is incorporated into the resulting fused seven-membered ring system (pictured below). According to the team, this heterocyclic structure had not been accessible so far and represents an entirely new scaffold.
The team studied the mechanism of this unusual reaction, which relies on electrophilic amide activation, and proposed a 5→7 ring enlargement as the key step. Due to the absence of a keteniminium intermediate, the stereogenic information at the α‐position is fully retained. The team will perform further studies on the reactivity of the seven-membered ring products.
.jpg)
- A Novel Class of 7-Membered Heterocyclic Compounds,
Adriano Bauer, Eszter Borsos, Nuno Maulide,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000363