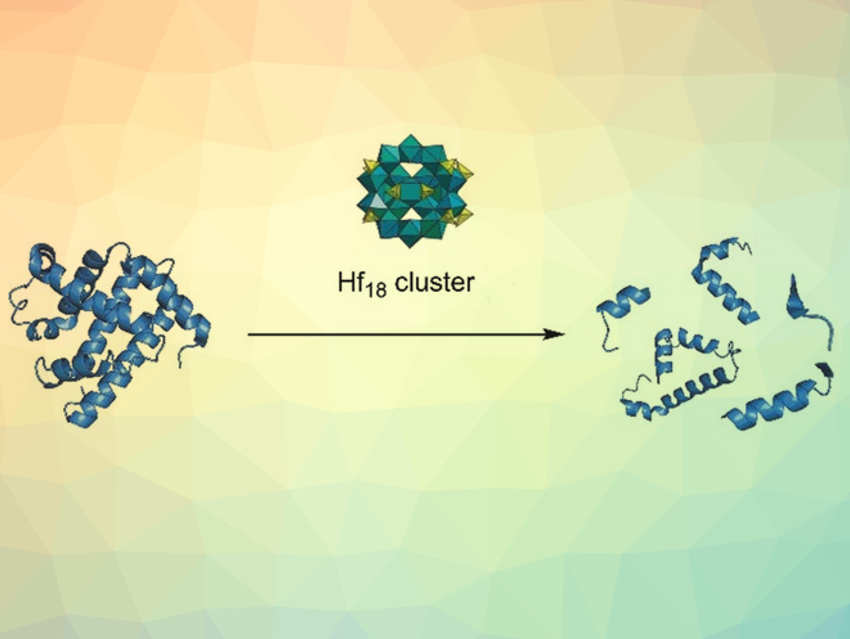
The selective hydrolysis of peptide bonds is important for protein research and analysis. Proteolytic enzymes are typically used for this type of hydrolysis reaction. However, they are limited by their high cost and sensitivity to small changes in the reaction conditions. Non-enzymatic catalysts could be an attractive alternative, but are challenging to develop.
Tatjana Parac-Vogt, KU Leuven, Belgium, and colleagues have tested the peptidase activity of the solid metal-oxo cluster (MOC) [Hf18O10(OH)26(SO4)13·(H2O)33]. This Hf18 cluster readily precipitates from solutions containing H2O2, H2SO4, and HfOCl2. The solid cluster was suspended in a solution of horse heart myoglobin (HHM) to test its catalytic activity. Remarkably, myoglobin, a 154-amino-acid protein, was cleaved selectively at only six aspartate residues by the MOC.
Mechanistic studies focused on the hydrolysis activity of the cluster showed a combined effect of the Lewis-acidic Hf4+ sites and the Brønsted acidity of the cluster surface. According to the researchers, the observation of selective protein hydrolysis mediated by a discrete metal-oxo cluster and the tunability of MOCs suggest that new heterogeneous nanozymes that mimic proteases could be developed. Nanozymes are nanomaterials with enzyme-like properties.
- Discrete Hf18 metal‐oxo cluster as a heterogeneous nanozyme for site‐specific proteolysis,
Tatjana N. Parac-Vogt, Jens Moons, Francisco De Azambuja, Jelena Mihailovic, Karoly Kozma, Katarina Smiljanic, Mehran Amiri, Tanja Cirkovic-Velickovic, May Nyman,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202001036