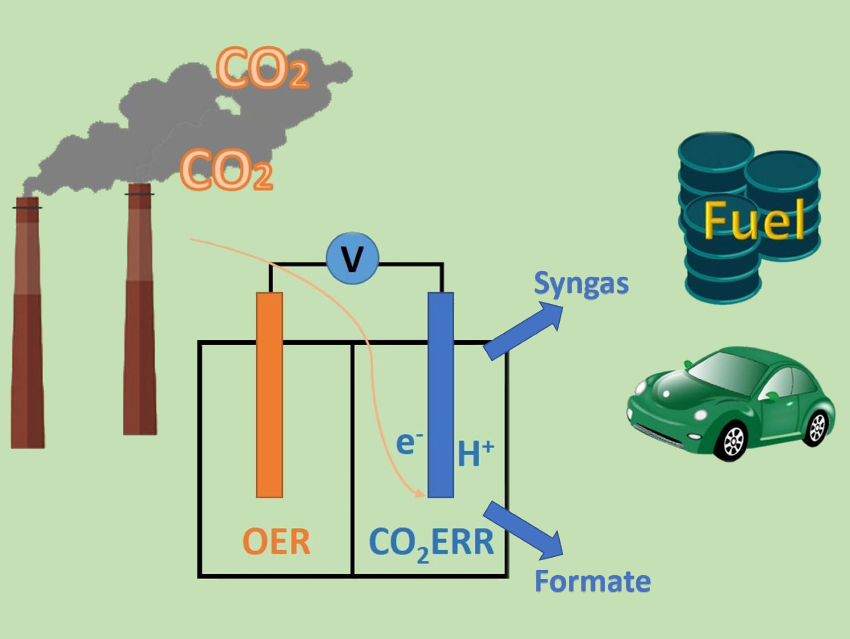
The electrochemical CO2 reduction reaction (CO2ERR) could enable environmentally friendly carbon utilization and value-added chemicals production. Formate and syngas (a mixture of hydrogen and carbon monoxide) are two typical products of the CO2ERR. They can be used as hydrogen storage materials and chemical feedstocks for the Fischer–Tropsch synthesis of hydrocarbons, respectively. The co-production of these two products maximizes the efficiency of electrochemical CO2 conversion.
Jinlong Gong, Tianjin University, China, and colleagues have synthesized ultrasmall SnO2 nanodots (NDs) homogeneously anchored on carbon nanotubes (CNT#SnO2 NDs) to be used as electrocatalysts for the CO2ERR process. The CNT#SnO2 NDs were prepared from COOH‐functionalized CNTs and SnCl4⋅5 H2O in an aqueous solution of glycine, hydrochloric acid, and ethylene glycol using ultrasonication.
The obtained CNT#SnO2 NDs can be coupled with oxygen evolution reaction (OER) catalysts. This results in a CO2ERR-OER electrolyzer with a low driven voltage of approximately 2 V. It can be used for the long-term co-production of formate and syngas. The high activity and selectivity of this system are stable for up to 24 h.
- Selective Electro-Reduction of Carbon Dioxide over SnO2-Nanodot Catalysts,
Jinlong Gong, Congling Hu, Lulu Li, Wanyu Deng, Gong Zhang, Wenjin Zhu, Xintong Yuan, Lei Zhang, Zhi-Jian Zhao,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202000557