The conversion of C-OH bonds to C-H bonds, or deoxygenation, is an important process in organic synthesis. It can be used for the transformation of high-volume, low-value biomass into high-value chemicals and fuels. Existing protocols for deoxygenation generally use environmentally harmful metals as catalysts. The development of metal-free pathways for direct and selective deoxygenation reactions would, thus, be useful.
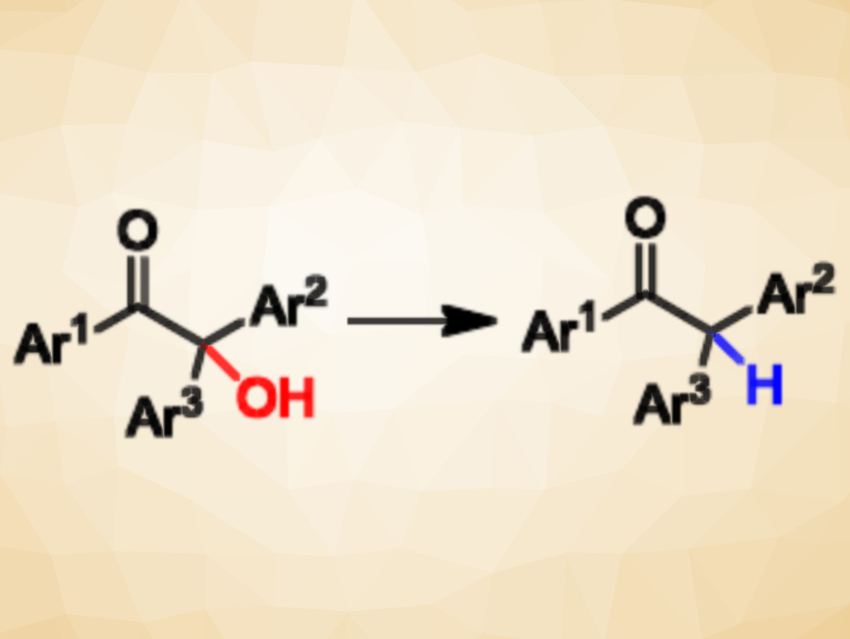
Anil Kumar, Panjab University, Chandigarh, India, and colleagues have developed a metal-free, direct, and selective approach for the deoxygenation of 2-hydroxy-1,2,2-triarylethanones to access 1,2,2-triarylethanones (pictured). The team used low-cost aqueous HClO4 as a Brønsted acid catalyst and triethylsilane (Et3SiH) as a soluble hydride source. The reaction was performed at 40 °C in CH2Cl2 and the desired products were obtained in good to excellent yields.
The researchers have also used the protocol to deoxygenate α-hydroxy acids and α-hydroxy esters. Mechanistically, the team proposes the involvement of an α-carbonyl carbenium ion as an intermediate, which is then trapped by the hydride source to give the deoxygenated product.
- Metal Free, Direct and Selective Deoxygenation of α-Hydroxy Carbonyl Compounds: Access to α,α-Diaryl Carbonyl Compounds,
Mr. Sandeep, Paloth Venugopalan, Anil Kumar,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000142

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

