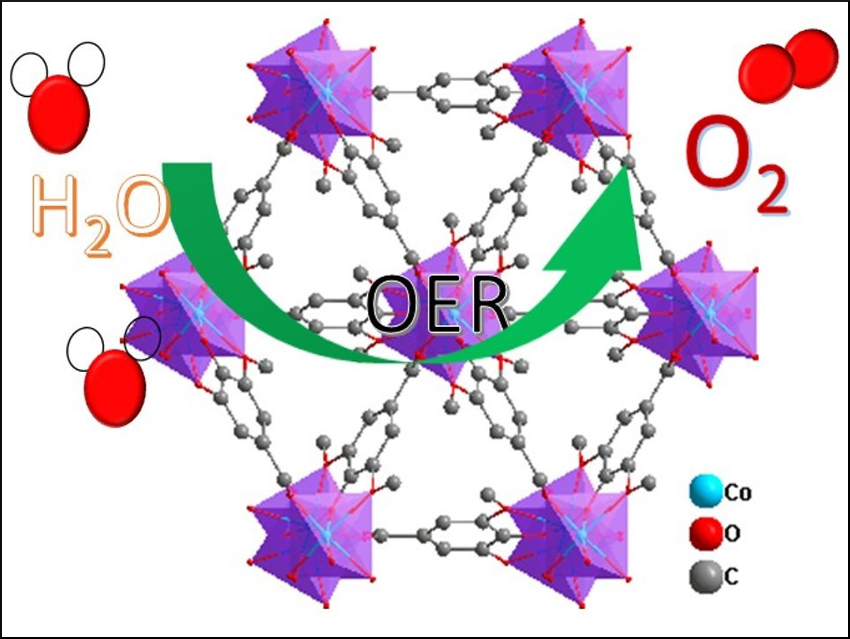
Hydrogen production via water splitting is a promising path to clean energy. However, this conversion suffers from a kinetically sluggish half-reaction: the oxygen evolution reaction (OER). It is, thus, important to find active and durable earth-abundant OER electrocatalysts to improve the overall efficiency of water splitting.
Jun-Ling Song, Jiangnan University, Wuxi, China, and colleagues have developed an efficient N,S-co-doped electrocatalyst for the OER based on a supermicroporous CoFe-based metal–organic framework (MOF) nanomaterial that can be quickly synthesized in high yield using a one-pot solvothermal reaction. The team used thiourea as both a nitrogen- and a sulfur source for doping, syringic acid (SyA or 4-hydroxy-3,5-dimethoxybenzoic acid) as a ligand, and CoCl2 and FeSO4 to prepare the MOF in dimethylformamide (DMF) at 150 °C.
This resulting catalyst shows good performance with a low overpotential and long-term electrochemical durability (at least 16 hrs) for the OER in alkaline media. The high performance is attributed to the N,S-co-doping, which facilitates the formation of active species. According to the researchers, this enables the direct use of MOF nanomaterials as catalysts for the OER and could also provide a new strategy for the design of other highly efficient hetero-doped MOF catalysts.
- Rational Design of a N,S co-Doped Supermicroporous CoFe-Organic Framework Platform for High-Efficient Water Oxidation,
Jun-Ling Song, Zhao-Qian Huang, Bin Wang, Dong-Sheng Pan, Ling-Li Zhou, Zheng-Han Guo,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202000376