Ultrafast Repeated Staining and Destaining of Cell Samples
In the treatment of tumors, their microenvironment plays an important role. It often contains immune cells that are changed and promote tumor growth. Jonathan C. T. Carlson, Ralph Weissleder, Massachusetts General Hospital Research Institute and Harvard Medical School, both Boston, MA, USA, and colleagues have introduced a method by which cell samples from tumors and their surroundings can rapidly (under 1 hour) be cycled through staining, destaining, and then restaining with fluorescent antibodies—through the attachment of a “black hole quencher” (fluorescence quencher) by means of click chemistry.
To effectively and precisely fight a tumor, it is important to specifically characterize not only the cells of the tumor but also those in its microenvironment, including tumor-infiltrating immune cells. Until now, analyses of these dynamic changes with conventional biopsies and tissue sections could take days to weeks, or not occur at all before treatment. One alternative method is fine-needle aspiration, in which only a few thousand cells are taken from different parts of a tumor and its surroundings. This method has few risks and is faster because it does not require embedding or sectioning. However, to obtain a representative estimate of the immune cell populations in the tumor’s microenvironment, many different stains must be carried out. Because the number of cells is so small, this means that the same sample must be repeatedly stained, destained, and stained again. However, the cells are too delicate for conventional, harsh destaining, and the procedures would take too long.
Cyclic Method for Rapid Immune Cell Characterization
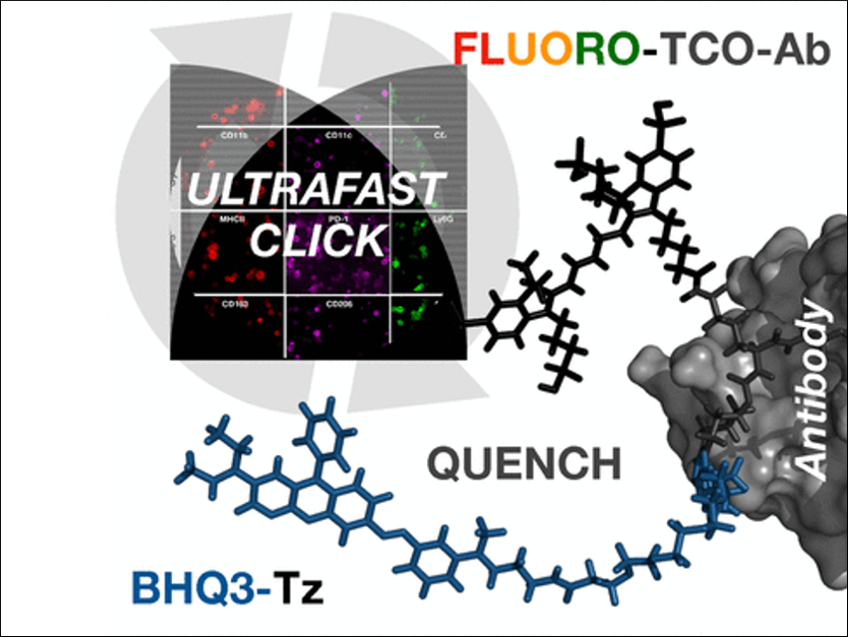
The team has developed an ultrafast, highly efficient, and gentle cyclic method for multiplexed protein profiling of individual cells, which allows for numerous different stainings. Instead of splitting off the dye or bleaching it, the fluorescence of the stain is simply “switched off” with a black hole quencher. Black hole quenchers absorb the energy of a fluorescence dye over the entire visible spectrum and convert it to heat as soon as they get near enough. This switches off the glow of the dye.
The method goes like this: using a connector that contains a trans-cyclooctene group, a fluorescent dye is attached to antibodies that specifically recognize the characteristic marker molecules of the cells. If the target marker is in a given sample, the antibody binds to it and the fluorescence can be detected. Then the quencher carrying a tetrazine group is added. Using this tetrazine group and the trans-cyclooctene, the quencher can simply be attached by being “clicked” on. The quencher is, thus, site-specifically and very quickly and efficiently brought near to the dye, immediately quenching its fluorescence. The rapidity of this click reaction is remarkable, running orders of magnitude faster than expected. The reason for this may be the strong interaction between the fluorescence dye and the quencher.
The next fluorescent antibody can be applied immediately after the fluorescence quenching. The researchers were able to stain twelve different marker molecules in a sample within one hour. This makes it possible to rapidly characterize the immune cell populations in tumors to select the most suitable treatments.
- Ultra-fast Cycling for Multiplexed Cellular Fluorescence Imaging,
Jina Ko, Juhyun Oh, Maaz S. Ahmed, Jonathan C. T. Carlson, Ralph Weissleder,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201915153