Cyanation reactions are important in organic synthesis. The use of non-toxic cyanation agents can provide improved safety and easier handling compared to conventional cyanide sources. In addition to their toxicity, these traditional reagents can have issues such as high costs, or moisture sensitivity.
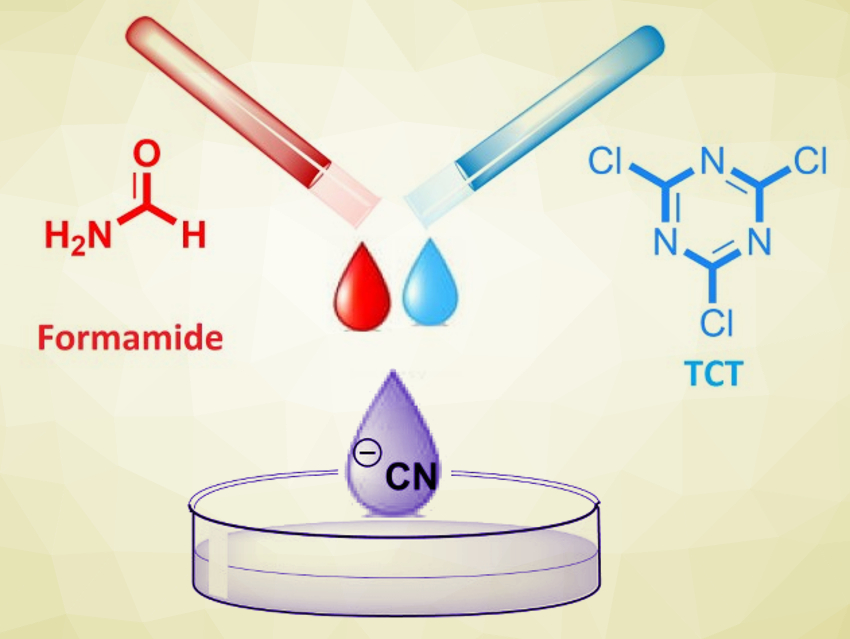
Farhad Panahi, Shiraz University, Iran, and colleagues have developed a new “CN” source using formamide and 2,4,6-trichloro-1,3,5-triazine (TCT), also called cyanuric chloride (pictured in blue). The Pd-catalyzed cyanation of aryl halides was successfully performed in the presence of this reagent mixture. The team used Pd/C as a catalyst, triphenylphosphine (PPh3) as a ligand, and potassium carbonate (K2CO3) as a base. The reaction was performed at 130 °C in formamide.
Using this method, different benzonitrile derivatives were synthesized in good to excellent yields. Overall, the cyanation reaction in the presence of formamide and TCT is an expedient and safe process for the cyanation of aryl halides. The reaction can be performed in two ways, either via an in situ approach or using a pre-formed TCT–formamide salt.
- Palladium-Catalyzed Cyanation of Aryl Halides using Formamide and Cyanuric Chloride as a New “CN” Source,
Esmaeil Niknam, Farhad Panahi, Ali Khalafi-Nezhad,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000117



Ammonium formate gives formaide on heating at elevated temperature and hydrogen cyanide using metal catalyst . Rhuthenium palladium.
Cyanuric acid in DMF gives good reagent which gives amino alkylation.
How mechanism proceeds? . It’s forms from imine or there is ammonia source.