Ruthenium-catalyzed C–H activation reactions allow the preparation of a variety of compounds that are useful, e.g., in materials science, the agrochemical industry, or medicinal chemistry. In electrochemical C–H activations, the use of (sustainable) electricity as a supply for redox equivalents can replace chemical oxidants. However, an in-depth mechanistic understanding of ruthenium electrocatalysis is still lacking.
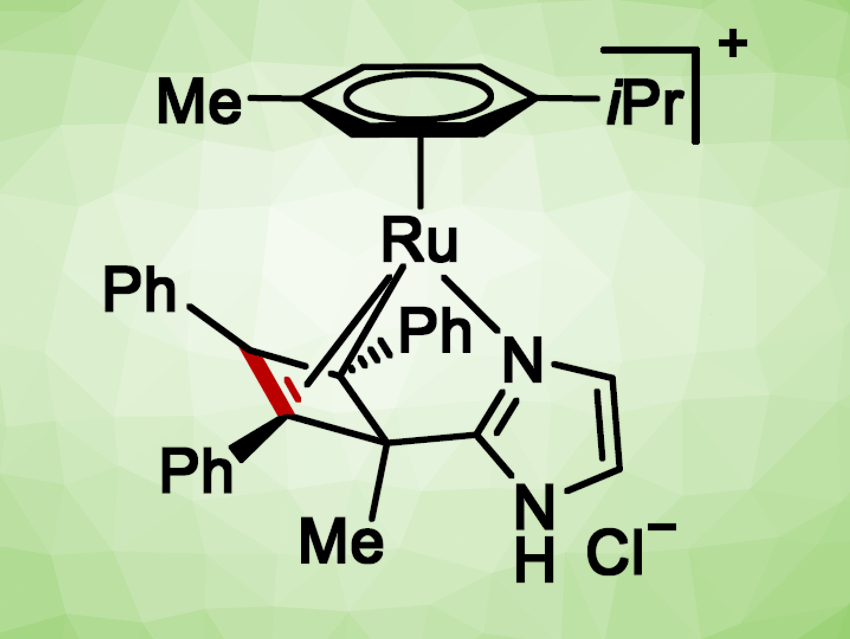
Lutz Ackermann and colleagues, University of Göttingen, Germany, have developed a ruthenium-catalyzed electrochemical dehydrogenative annulation reaction of imidazoles with alkynes (pictured below, GF = graphite felt). The reaction gives various bridgehead N−fused [5,6]-bicyclic heteroarenes. The team identified azaruthena(II)-bicyclo[3.2.0]heptadienes as unexpected intermediates in the reaction. These complexes, which were characterized by X-ray crystallography, are catalytically active.
Based on these findings, as well as density functional theory (DFT) calculations, the team proposes a mechanism that involves an oxidation-induced reductive elimination pathway and a ruthenium(II/III) catalytic cycle. These mechanistic findings could help to guide the design of new ruthenium-catalyzed oxidative C–H activations.

- Aza-Ruthena(II)-Bicyclo-[3.2.0]-Heptadiene: Key Intermediate for Ruthenaelectro(II/III/I)-Catalyzed Alkyne Annulations,
Long Yang, Ralf Steinbock, Alexej Scheremetjew, Rositha Kuniyil, Lars H. Finger, Antonis M. Messinis, Lutz Ackermann,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202000762