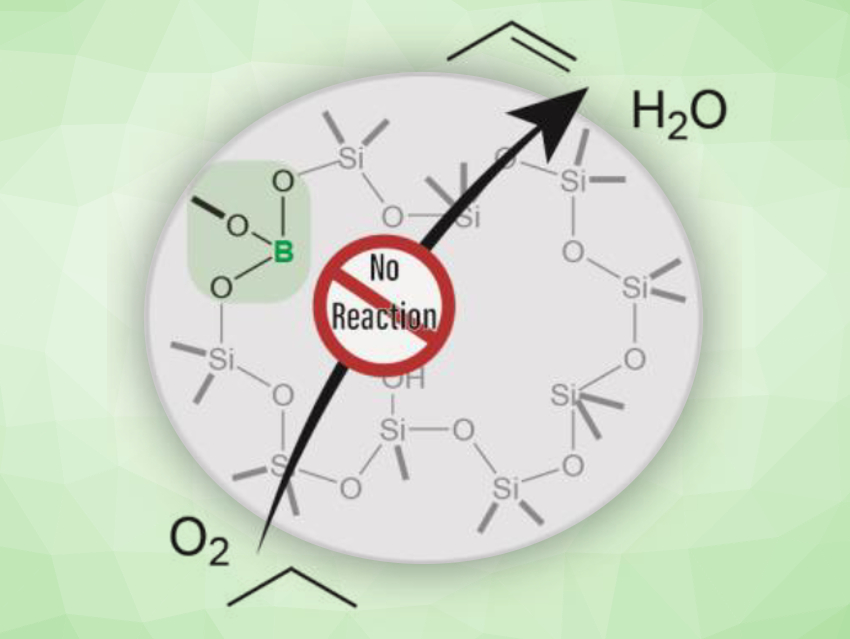
The oxidative dehydrogenation (ODH) of light alkanes is a promising and relatively low-energy method to produce important chemical building blocks such as ethylene and propylene. Boron-containing materials are highly selective catalysts for ODH. They produce minimal quantities of over-oxidation products like CO or CO2. A boron oxide phase is thought to be responsible for the catalytic activity of these materials; however, a defined active site had not been identified so far.
To understand the activity of these catalysts, Aaron J. Rossini, Iowa State University, Ames, USA, Ive Hermans, University of Wisconsin–Madison, USA, and colleagues have created single BO3 sites inside a silicate zeolite. This zeolite, called B-MWW, was hydrothermally synthesized. Catalytic testing showed that this material was not an active catalyst for the ODH of propane (pictured). This result is surprising, as a catalyst with the same boron loading, but supported on amorphous silica, was highly active for the same reaction.
Using solid-state 11B NMR spectroscopy, the team found that boron does not aggregate in the inactive zeolite-based model catalyst—in contrast to the active silica-supported catalyst. The researchers believe that aggregated boron sites are necessary for catalytic activity. This work challenges the traditionally favored hypothesis that isolated BO3 units are the active site in boron‐based catalysts
- B-MWW Zeolite: The Case Against Single-Site Catalysis,
Natalie R. Altvater, Rick W. Dorn, Melissa C. Cendejas, William P. McDermott, Brijith Thomas, Aaron J. Rossini, Ive Hermans,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201914696