Twisted Building Blocks
The controlled synthesis of porous solids is important in chemistry and materials science. Junliang Sun, Peking University, Beijing, China, and Stockholm University, Sweden, Cheng Wang, Wuhan University, China, and colleagues have taken a step towards the rational construction of covalent organic frameworks (COFs) based on twisted building blocks.
Highly porous, crystalline organic polymers are a relatively new class of materials and are commonly known as covalent organic frameworks (COFs), analogous to their chemical cousins, the metal–organic frameworks (MOFs). The potential of porous solids is almost always based on their large surface area relative to their volume. They can open up possibilities in catalysis, separation science, gas storage, energy storage, optoelectronics, and sensor technologies, where small target molecules might be adsorbed on to that vast surface in large numbers.
COFs are usually either layered two-dimensional structures or more conventional three-dimensional structures. Scientists have constructed a wide range of COFs in the 2D class, but so far, 3D COFs are rare. However, it is in the 3D type that we might see enormous surface-area-to-mass ratios—as high as 5,000 m2 per gram of material. It seems, however, that synthesizing these 3D forms is a lot harder than creating stacks of 2D materials. The researchers hoped to remedy that.
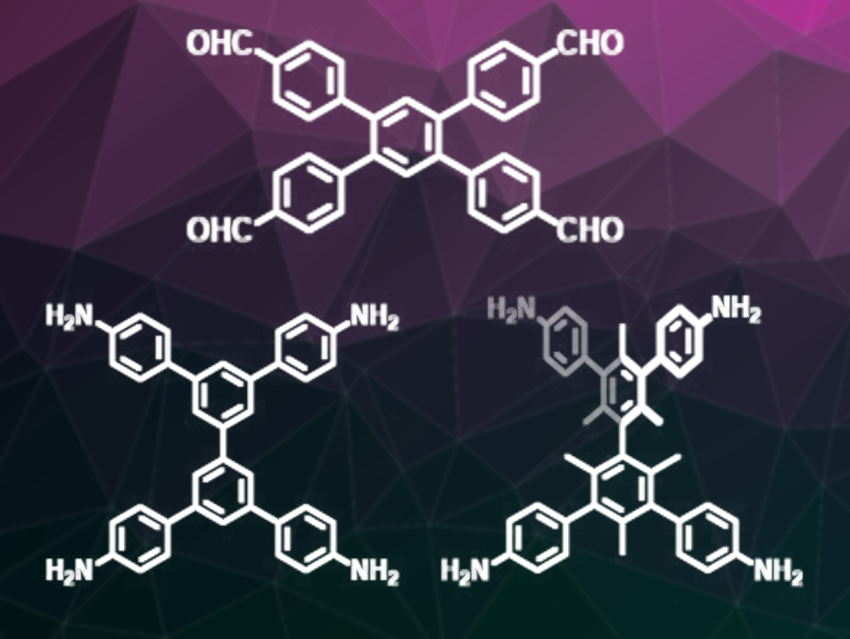
The team’s approach is to start with tetrahedral and quadrilateral building blocks (pictured) that can be connected through [4 + 4] condensation reactions. They have carried out the rational synthesis of two COFs, 2D-BPTA-COF and 3D-BMTA-COF, starting with biphenyl-based precursors with different groups at the ortho positions on the building blocks. BPTA is 3,3’,5,5’-tetra(p-aminophenyl)-biphenyl (pictured bottom left) and BMTA is 3,3’,5,5’-tetra(p-aminophenyl)-bimesitylene (pictured bottom right).
From Planar to Tetrahedral
The team explains that the 2D-BPTA-COF crystallizes into 2D sheets, adopting an AB stacking mode. By contrast, the 3D-BMTA-COF assumes a sevenfold inter-penetrated structure. This difference results from the presence or absence of the methyl groups in the building blocks. The dihedral angle of the biphenyl rings in BPTA is 0 degrees and gives rise to the 2D structure. In contrast, the methyl groups in BMTA twist this angle to 60 degrees and lead to a 3D structure. The team concludes from this finding that it should be “possible to synthesize COFs with different structures by twisting building blocks from planar to tetrahedral with steric hindrance”.
“This is the first study of the effect of reinforced non-planarity on the synthesis of COFs, and moreover, we have demonstrated a general and straightforward way to expand the diversity of tetrahedral nodes for the construction of other 3D COFs,” Cheng told ChemViews Magazine. “This is really important, as there are very few tetrahedral nets in the literature.”
“It is an interesting study,” Yi Liu of the Lawrence Berkeley National Laboratory, University of California, USA, told us. “The team reported a clever way to construct 3D COFs from a biphenyl linker, the conformation of which was dictated by steric hindrance of methyl groups to be a multivalent tetrahedron,” He points out that this 3D structure is in “stark contrast” to the more conventional 2D COF structures formed when a steric-free biphenyl linker is employed instead.
“This is an elegant example demonstrating that small structural change can lead to a drastically different outcome when exercising reticular chemistry in COF synthesis,” he adds. “The study also suggests that there is more flexibility when choosing building blocks—most building blocks of 3D COFs are more rigid than the biphenyl used in this study.”
- Twist Building Blocks from Planar to Tetrahedral for the Synthesis of Covalent Organic Frameworks,
Chao Gao, Jian Li, Sheng Yin, Junliang Sun, Cheng Wang,
J. Am. Chem. Soc. 2020, 142, 3718–3723.
https://doi.org/10.1021/jacs.9b13824

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

