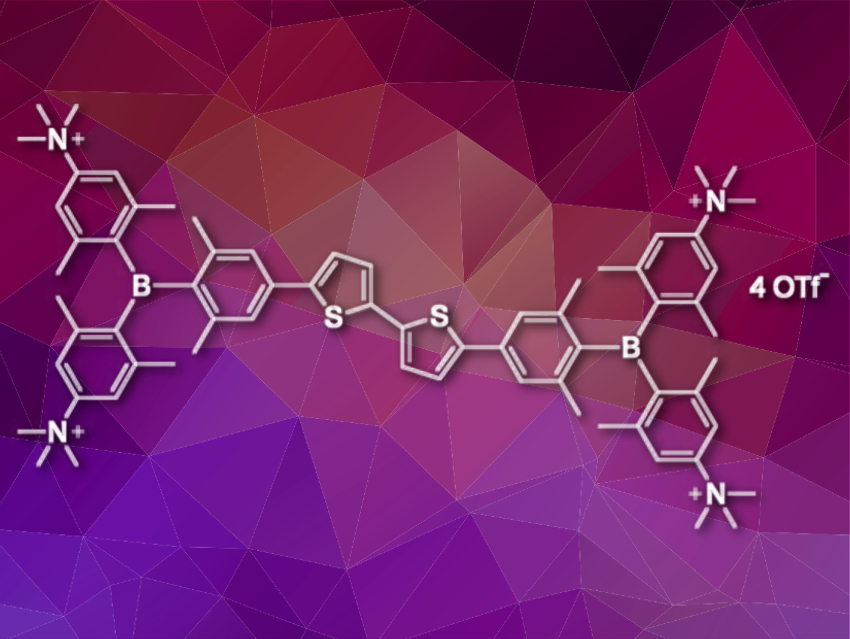
Bioimaging is an essential tool in biochemical and biomedical research, and there is a constant search for new small-molecule probes. Triarylborane chromophores are typically unstable and insoluble in water and, thus, until recently, they were not used in bio-related applications. Quadrupolar tetracationic bis-triarylborane chromophores (pictured), however, are both soluble and stable in water. They feature para-trimethylammonium substituents on the outer aryl rings to achieve water solubility. The ortho-methyl groups on all aryl rings provide steric protection for the empty p-orbital at boron that serves as a π-acceptor in the chromophore.
Ivo Piantanida, Rudjer Boškovic Institute, Zagreb, Croatia, Todd B. Marder, University of Würzburg, Germany, and colleagues have demonstrated that these water‐soluble tetracationic quadrupolar bis‐triarylborane dyes can bind exceptionally strongly (with 10-100 nM affinity) to DNA, RNA, and proteins. The team chose several types of DNA and RNA for their study, including naturally occurring calf-thymus DNA, synthetic alternating polynucleotides, single‐stranded synthetic ss‐RNA polynucleotides, as well as the most naturally abundant protein, bovine serum albumin (BSA).
The researchers found that the dyes can be used as a dual luminescence probe, efficiently distinguishing between DNA/RNA and protein (BSA) by characteristic fluorescence emission points separated by 3600 cm–1. These well-separated emissions allowed the simultaneous quantification of DNA/RNA and BSA in a mixture.
- A Quadrupolar Bis‐Triarylborane Chromophore as a Fluorimetric and Chirooptic Probe for Simultaneous and Selective Sensing of DNA, RNA and Proteins,
Željka Ban, Stefanie Griesbeck, Sanja Tomić, Jörn Nitsch, Todd B. Marder, Ivo Piantanida,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201903936