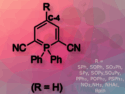
Helicenes are polyaromatic organic molecules that adopt a corkscrew-like conformation. For this reason, there are two possible enantiomeric forms of each structure. In small helicenes, such as [4]helicenes, both enantiomers easily interconvert. However, this racemization can be avoided with suitable substituents at the “inner” positions.
Manuel Alcarazo, University of Göttingen, Germany, and colleagues have performed the first highly enantioselective synthesis of 1,12‐disubstituted [4]carbohelicenes. The team used various aromatically substituted alkynes (pictured above) as substrates and performed an intramolecular hydroarylation using gold catalysts (pictured below) to prepare a range of [4]helicenes in enantiopure form. The catalysts are based on an α-cationic phosphine which contains a TADDOL unit as a chiral inductor (TADDOL=α,α,α,α‐tetraaryl‐1,3‐dioxolane‐4,5‐dimethanol).
The absolute stereochemistry of the prepared structures was determined by X‐ray crystallography. The products could have applications as functional materials with useful electrooptical properties.
.jpg)
- Enantioselective Synthesis of 1,12-Disubstituted [4]Helicenes,
Manuel Alcarazo, Thierry Hartung, Rafael Machleid, Martin Simon, Christopher Golz,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201915870
![Conformationally Stable [4]Helicenes](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/170778f7bf8.jpg)