High-quality metalloenzyme crystals are important for the structure-based design of biocatalysts. However, preparing bulk quantities of metalloenzyme microcrystals is challenging. Cytochrome P450 102A1 (or P450BM3), for example, has a high catalytic activity and is a promising candidate for use as a versatile biocatalyst. When this enzyme is modified to change its reactivity, it can be almost impossible to obtain high‐quality crystals suitable for X‐ray crystallography.
Hiroshi Sugimoto, RIKEN SPring-8 Centre, Sayo, Japan, Science and Technology Agency, Tokyo, Japan, Osami Shoji, Nagoya University, Japan, and Science and Technology Agency, Japan, and colleagues have used a crystallization accelerator to obtain crystals of the haem domains of P450BM3 variants. The team used the diterpenoid derivative N‐abietoyl‐L‐tryptophan (AbiATrp) to accelerate the crystallization of wild‐type, unmodified P450BM3. AbiATrp is a substrate analogue for the enzyme.
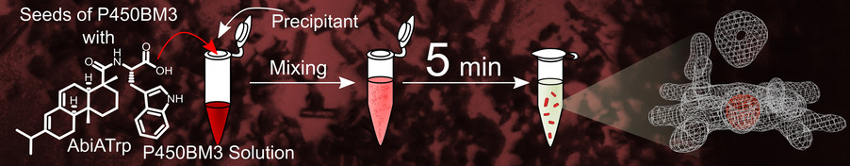
The resulting crystals were used as seeds to prepare microcrystals of a range of P450BM3 variants (example pictured above), including both substrate-free and substrate-analogue-bound variants, as well as heavily tagged variants and ones that contain synthetic metal complexes. Mixing the seeds of P50BM3-binding AbiATrp with the variants of P450BM3 resulted in microcrystallization within minutes (process pictured below). The microcrystals prepared by the method are suitable for analysis with X-ray free-electron lasers (XFEL), which allows the monitoring of reaction intermediates in real time.
- Crystals in Minutes: Instant On‐Site Microcrystallisation of Various Flavours of the CYP102A1 (P450BM3) Haem Domain,
Joshua Kyle Stanfield, Keita Omura, Ayaka Matsumoto, Chie Kasai, Hiroshi Sugimoto, Yoshitsugu Shiro, Yoshihito Watanabe, Osami Shoji,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201913407




