Sodium-ion batteries (SIBs) provide a promising alternative to lithium-ion batteries (LIBs). SIBs are generally both cheaper and more sustainable than LIBs because they use earth-abundant elements.
Xin Li, Harvard University, Cambridge, MA, USA, and colleagues have introduced Cu into cathode materials for SIBs, resulting in improved performance. The team synthesized a series of layered Na0.75Mn0.6Fe0.2(CuxNi0.2‐x)O2 compounds, using only low-cost, earth-abundant metals. The compounds were synthesized by solid‐state reactions starting from sodium carbonate, manganese oxide, iron oxide, copper oxide, and nickel oxide. The team found optimal capacity retention in the resulting batteries at a moderate Cu doping level.
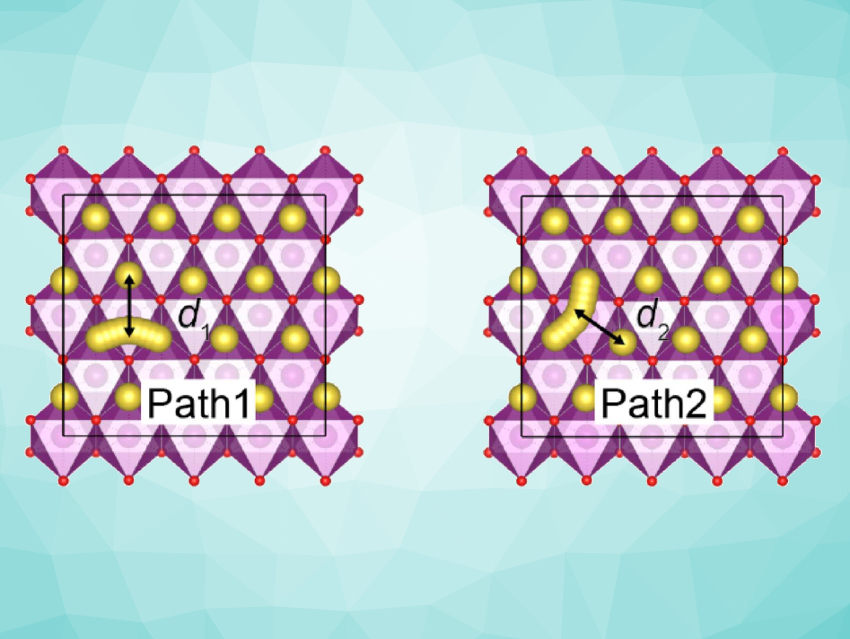
The researchers found that at a low voltage, Cu2+ can induce competing effects on the ionic and electronic conductivities, which is why medium copper levels result in optimal performance. The copper ions cause a Jahn‐Teller distortion, which enhances the Na-ion diffusivity (diffusion pathways pictured) and reduces the electronic conductivity. The work could help with the design of next-generation SIBs.
- A Study of Cu Doping Effects in P2‐Na0.75Mn0.6Fe0.2(CuxNi0.2‐x)O2 Layered Cathodes for Sodium‐Ion Batteries,
Yichao Wang, Sooran Kim, Jingyu Lu, Guangyuan Feng, Xin Li,
Batteries Supercaps 2020.
https://doi.org/10.1002/batt.201900172